The human sense of smell, while not exactly on par with that of many animals, is often marveled at for its acuity. Considerable research has gone into understanding the labyrinthine networks at play in olfactory processing and computation. Researchers in the Carlson Lab at Yale have recently shown, in a study published in Nature, that olfactory neurons in fruit flies (Drosophila melanogaster) may communicate through a method that was once considered an epiphenomenal manner of communication: ephaptic coupling.
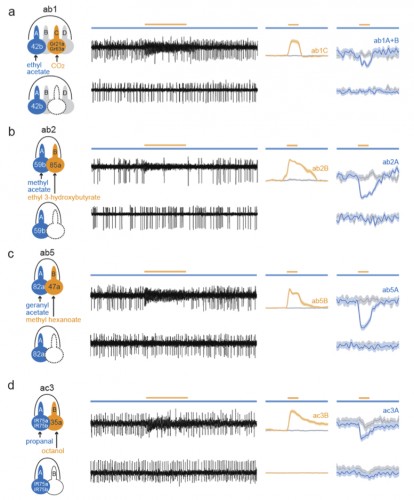
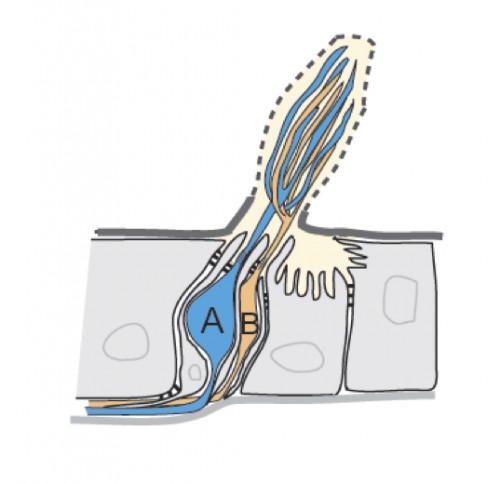
The surface of insect olfactory organs is covered with numerous sensory hairs called sensilla that contain olfactory receptor neurons. Those neurons detect odor molecules so as to transform chemical information (odorants) into electrical information (action potentials), a common language understood by the brain. A given sensillum can contain anywhere from one to four grouped olfactory neurons that are each tuned to respond to specific odorants. In the classical model of neuronal signaling an active neuron will signal to another neuron through chemical messengers (neurotransmitters) at specialized structures between neurons, called synapses, in order to propagate the message. Yet, as this study showed, insect olfactory neurons that share the same sensillum communicate with one another via another means: direct electrical interaction via ephaptic coupling.
For decades, scientists were skeptical that such direct electrical interaction was a biologically relevant means of neuronal communication; the occurrence was rare and the strength weak. However, as Dr. Chih-Ying Su, the primary author of the Nature study, put it, “in the case with olfactory sensillum, the limited and insulated space shared by the neighboring neurons is conducive for ephaptic interaction”.
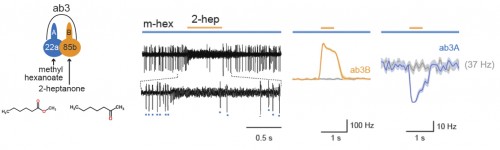
Using a two-odorant paradigm that “was used to mimic what insects encounter in nature,” Su and her colleagues found that transient activation of one neuron cross-inhibits the chronic response of its neighbor, a form of neuronal interaction known as lateral inhibition. Further experiments showed that such lateral inhibition within a sensillum could still occur even after synapse transmission from olfactory receptor neurons to the brain was blocked, either genetically or surgically. The same lateral inhibition also occurred in a type of malarial mosquito known as Anopheles gambiae, suggesting a broad occurrence of ephaptic interaction among insect olfactory sensilla. Furthermore, the authors showed that such ephaptic coupling is robust enough to modulate flies’ behavioral response to a binary odor mixture, in which each of the constituents activates one of the grouped olfactory receptor neurons in the same sensillum.
This study has shown that olfactory neurons in the same sensillum are, in themselves, a layer of computation at the periphery, which challenges the conventional view that all sensory information is processed solely in the brain. While it is not known if ephaptic coupling occurs in the human olfactory circuit yet, studying it could be quite challenging. For now, Su and colleagues believe that this newly-discovered method of neuronal communication in insects could be used to develop ways to manipulate the host-seeking behaviors of insects, which use their keen sense of smell to destroy crops and transmit diseases to humans.