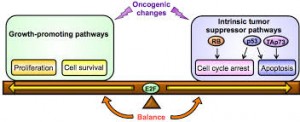
Cancer is a genetic disease. An accumulation of mutations within our DNA can lead to cell division and differentiation so rapid that our normal cells can scarcely keep up. For years, the malignance of these mutations was attributed to their ability to activate oncogenes, cancer-promoting sequences in our DNA that tell normal cells in our body to proliferate without reprieve. But we now understand that oncogene activity is nearly harmless without an initial switch — this first step opens the floodgates, weakening the cell so that oncogenes can fulfill their potential in causing tumors. New research led by Ruslan Medzhitov, a David W. Wallace professor of immunobiology, demonstrates this two-hit model in Yap, a protein heavily implicated in the development of liver cancer.
Published in February in the journal eLife, Medzhitov’s study sheds light on the oncogenic activity of Yap, a protein that aids in maintaining normal cell differentiation and survival. Prior to this research, little was known about the tumor suppressive mechanisms that safeguard against the growth-promoting activity of Yap. In cancer, Yap’s expression can increase so dramatically that the protein activates genes telling the cell to divide continuously and uncontrollably.
The Yale scientists used a mouse model in which Yap is overexpressed in a fraction of otherwise normal liver cells, called hepatocytes. The goal: determine how Yap is able to act as an oncogene. The group found that Yap activation alone is insufficient to spur tumor development in the liver. Rather, simultaneous tissue damage or inflammation is required to induce cancer-like proliferation of hepatocytes. In fact, Medzhitov’s work suggests that Yap expression can be an important mechanism in recovering from liver injury because of its ability to accelerate cell regeneration. Most importantly, these findings highlight the importance of a second signal in the cell that enables Yap to achieve an oncogenic phenotype. Otherwise, the Yap phenotype remains an important player in liver tissue repair.
This study also poses new research questions. Scientists may use this insight into Yap to develop anti-cancer therapies. The authors of the paper suggest that a protein called IL-6 aids in Yap-induced oncogenesis — targeting IL-6 could be an exciting opportunity to treat liver cancer, which affects hundreds of thousands of people every year. Finding ways to turn off the oncogenic potential of Yap would be a major stepping stone in developing improved treatments for liver cancer.
