Have you ever wondered what it would be like to eat all of the cookies you want without worrying about weight gain? It seems too good to be true—a fantasy, even. Yet, new research indicates that it may, indeed, be possible… at least in mice.
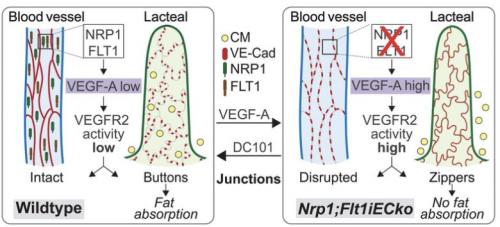
Yale Medical School researcher Professor Anne Eichmann and her team have discovered a brand-new mechanism that curbs weight gain. Eichmann’s team fed mice a high-fat diet, expecting to create a group of obese mice. But these mice simply did not gain weight. Her team located two cell receptors responsible for this lack of weight gain: neuropilin-1 (NRP1) and vascular endothelial growth factor receptor 1 (FLT1). When these receptors were inactivated, open pathways in fat-absorption vessels of the small intestines, also known as lacteals, were closed. This process resembles a zipper, with the absence of these genes causing zipping. But how, exactly, did these genes do that?
With the inactivation of these receptors, the gene VEGF-A grew in bioavailability and signaling through the receptor VEGFR-2 increased. This mechanism caused lacteal junction zippering, which caused poor fat absorption.
To confirm, Eichmann’s team deleted two genes coding for NRP1 and FLT1 in mice. This caused an increase in VEGF-A and a decrease in fat absorption. Additionally, Eichmann’s team injected VEGF-A into the body, observing an increase in zipper-like junctions in the lacteals.
After Eichmann’s team determined that a high VEGF-A concentration induced the zippering of lacteal junctions, they studied whether these changes impaired fat uptake. To do this, the team studied BECs (blood endothelial cells), which line blood vessels, and LECs (lymphatic endothelial cells), which line lymphatic vessels. Using an antibody that disrupts cell junctions, Eichmann’s team found that disruption of BEC junctions does not affect fat uptake, while disruption of LEC junctions decreases fat uptake.
Based on these findings, Eichmann’s team concluded that increasing VEGF-A signaling opens junctions in blood vessels, while closing junctions in lymphatic vessels. Even short-term increase of VEGF-A changes lacteal junction morphology, decreasing fat uptake through zippering. Additionally, NRP1 and FLT1 work together to lessen VEGF-A signaling, which explains the decrease in fat absorption when they are inactivated. An understanding of the mechanisms behind lacteal junction zippering in lymphatic vessels is integral for identifying molecular targets to treat obesity.
According to Feng Zhang, first author of this study and an associate research scientist on Eichmann’s team, the results of this study could be foundational for the future of diabetes treatment methods.
“Obesity and obesity-related metabolic diseases such as diabetes are major health concerns for patients and doctors. So far, there are not many effective approaches to stop diet-induced diabetes in the general population,” Zhang says. “Our finding may lead to a new approach to treat diet-induced diabetes.”
However, he believes that human trials are still in the distant future. Future studies into lacteal junction zippering and its potential effects on intestinal health are necessary to provide a fuller understanding of this pathway.