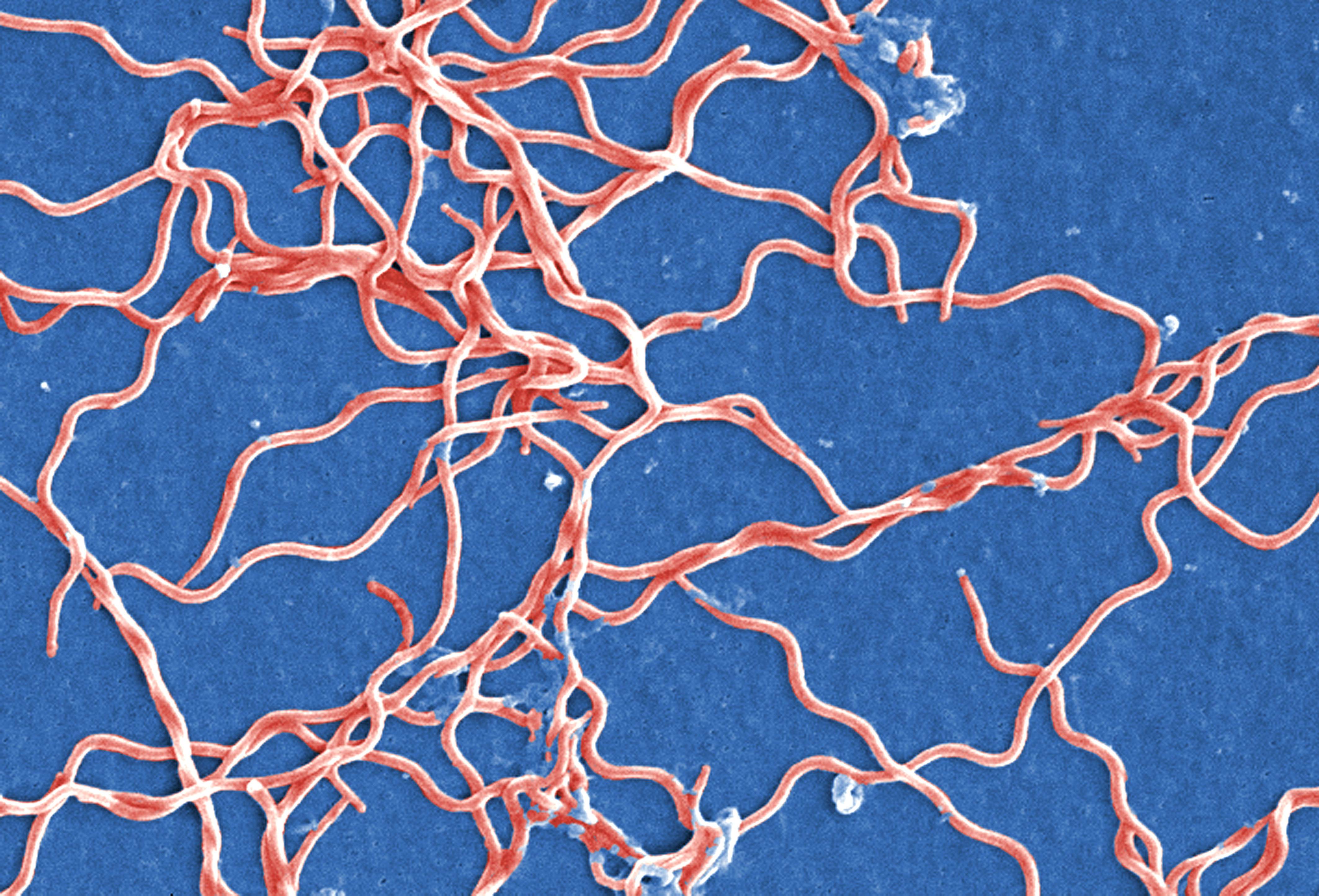
In a beauty contest of the bacterial world, spirochetes would certainly be the odd ones out. This bizarre lineage of bacteria, which includes the Lyme disease-causing agent Borrelia burgdorferi, is long, twisted, and spindly. Resembling pieces of noodle, spirochetes can be a hundred times longer than they are wide.
A recent study led by Yale microbiology professor Christine Jacobs-Wagner adds another item to B. burgdorferi’s list of oddities – the peculiar way in which they grow and divide. Her team found that the bacterium elongates in discrete “hot zones” of growth that mark where the next generation will divide. Given the growing threat of Lyme disease, this discovery sheds light on the unique biology of this pathogen and could suggest important therapeutic targets.
According to Molly Scott, a fourth-year graduate student in the Yale Department of Molecular, Cellular and Developmental Biology and one of the study’s first authors together with postdoctoral associate Brandon Jutras, the group’s interest in B. burgdorferi was sparked by the surprising lack of knowledge about the bacteria’s basic biology.
“Borrelia burgdorferi has been studied a fair amount since it was discovered as the causative agent of Lyme disease, but most people have focused on how it causes disease, looking at how the bacteria survives in tick vectors and mammalian hosts during its life cycle,” Scott said. “Yet we don’t actually understand much about the basic cell biology of the organism,” she added.
In particular, not much is known about how B. burgdorferi carries out the most fundamental biological processes – growth and division. This question is particularly interesting given B. burgdorferi’s unusual length. “The fact that Borrelia burgdorferi looks so fundamentally different from other bacteria raises a lot of fascinating questions about how it is able to regulate this incredible space conundrum,” Scott said.
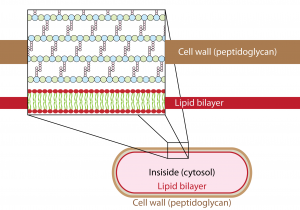
For bacteria to grow, they need to expand their cell wall, a spandex-like structure composed up of interlacing units called peptidoglycan that surrounds the cell. Most bacteria grow one of two ways. Some, like Escherichia coli, grow by incorporating additional peptidoglycan – more spandex – along their entire length. Others opt for a different route, continuously tacking peptidoglycan onto just one of their ends in order to grow.
But when the Jacobs-Wagner team looked at B. burgdorferi, they found something completely different. To visualize growth, the researchers used a fluorescent version of a peptidoglycan unit, such that peptidoglycan newly added to the cell wall would glow green. What they saw was striking – rather than be spread out evenly over the entire bacteria, or pushed to one end, the green newly added peptidoglycan was mainly concentrated in a few very bright regions across the bacteria. It seemed that the bacteria were growing with discrete “hot zones” of highly active cell wall synthesis – a relatively quiet construction zone with just a few buzzing areas of activity, workers scurrying about. “This is something fundamentally different from what we’ve seen before, and not at all what we were expecting,” Scott said.
The team was curious whether this pattern of bacterial cell growth was related to at all to the process of cell division. “We wondered to what degree these processes overlap and perhaps talk to each other,” Scott said. They discovered that a bacterium is born with a single “hot zone” of cell wall synthesis at its center (the “1/2 zone”). As the cell is preparing for division, it develops new hot zones at sites ¼ and ¾ of its length. This means that when the cell splits in two, its daughter cells will inherit these ¼ and ¾ zones, marking where they will eventually divide and beginning the cycle anew. In this way, a mother cell establishes where its daughter cells will divide – an overprotective parent at their finest.

Yet how do the bacteria “know” where to place the ¼ and ¾ zones? According to Scott, one option is that the bacteria grow some set length – say, ten microns – and then establish a new zone of synthesis. Alternatively, rather than determine an absolute length, the bacteria may sense the relative positions of the ¼, ½, and ¾ marks. To explore this question, the team looked at B. burgdorferi strains with a wide range of lengths, and found that the pattern of “hot zones” stationed at the ¼, ½, and ¾ lengths was always consistent. “This indicates that it is not that the cells are just growing a set length and defining a new zone,” Scott said. “They are really able to sense the relative locations somehow,” she added.
The mechanism underlying this sensing is not entirely clear. According to Scott, the genome of B. burgdorferi lacks genes that many other types of bacteria use for their cell division processes, complicating efforts to pinpoint a mechanism. Moving forwards, the group hopes to probe this mechanism further. Scott is also particularly interested in exploring how the location of DNA in the cell could regulate cell division in B. burgdorferi, another active area of research.
In the long run, the bizarre nature of this discovery may actually be the reason why it has value in the clinic. One of the major challenges of antibiotics is that they often act on processes common to many bacteria – thus sweeping out the “good” micro-organisms with the bad. If researchers can find processes specific to B. burgdorferi and B. burgdorferi only – like its strange mode of cell growth – then they can design drugs to target this process that will not harm other types of microbes. “As soon as we can find major differences between bacteria, then it suggests therapeutic targets,” Scott said, adding, “We are a while away from that, but it is exciting.”