When you eat sugar, your body does more than enjoy the sweet taste: it gets right to work breaking down the sugar molecules, digesting the starch that you find in foods such as potatoes into tiny molecules called glucose, a form of sugar that your body can convert into energy. If there is too much sugar in the bloodstream, insulin is responsible for transferring glucose from the bloodstream into cells. A disruption to this system, however, has drastic consequences.
Researchers at Yale University, led by Kevan Herold, Professor of Immunobiology, studied Type I diabetes, an autoimmune disorder in which a body’s own immune system turns against itself, targeting its own beta cells. These beta cells are found in the pancreas and produce the hormone insulin, which normally responds to changes in levels of glucose in the blood. In some individuals with Type I diabetes, the immune system destroys its own beta cells. However, a number of beta cells manage to evade these self-killing mechanisms. Herold and his team’s study focused on this unexplained question of beta cell survival. Their research presented an astonishing discovery: a unique subpopulation of beta cells emerges following diabetes onset that show prolonged survival to immune attack. These findings provide hope for those with Type I diabetes.

A NOD in the right direction
Despite advancements in research on Type I diabetes, much remains unknown. Specifically, it is unclear why beta cells are killed off at different rates in Type I diabetics. In addition, why these cells are not totally destroyed in some individuals with Type I also remains unclear. To better understand the changes that occur at the molecular level during diabetes onset, the researchers studied non-obese diabetic (NOD) mice, a strain of mice that spontaneously develops diabetes at around 10 weeks of age, and which are considered the gold standard for studying diabetes.
Researchers noticed that among four-week-old NOD mice, a small population of beta cells expressed fewer glucose containing pockets than other beta cells did. Even more notably: as time progressed, the population of these lower-glucose cells increased compared to other types of beta cells, comprising up to half of all beta cells by the time the mice were 12 weeks old. The researchers found that these so-called “bottom beta cells” produced less insulin than other beta cells and responded less to the presence of glucose. But just what were these cells, and what was their function?
Getting to the Bottom of it
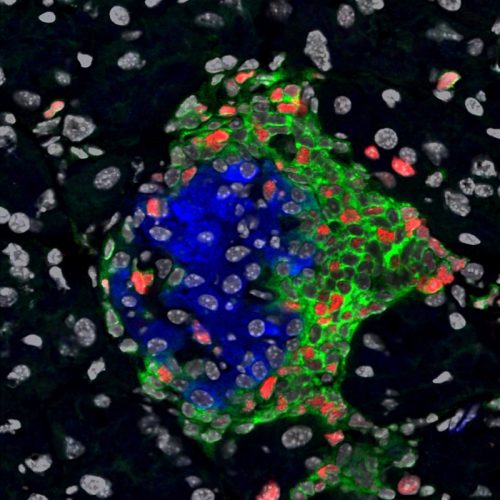
To confirm that these bottom beta cells were still functioning, researchers tested their ability to produce insulin, the defining characteristic of beta cells. They inserted the insulin gene into NOD mice along with a fluorescent gene. The fluorescent gene emitted a green light, enabling the scientists to detect insulin production. The intensity of fluorescent light would correlate to the amount of insulin produced. Both non-diabetic and Type I diabetic mice displayed fluorescent light, indicating that they still produced insulin. “This means cells are transcribing insulin, which we took to mean they are beta cells,” said Herold.
Having determined that the bottom beta cells really were beta cells, the researchers were interested in studying how they differed from normal beta cells at the genetic level. To test this, they sequenced the genes of both normal beta cells and bottom beta cell populations, identifying 457 genes in which differences were found between populations. What they found was astonishing: bottom beta cells displayed traits characteristic of stem cells, capable of proliferating and differentiating into various specialized cell types. “This raises a question that maybe these cells represent another cell, such as a stem cell, and have now acquired qualities of a beta cell,” Herold said.
Unlike other cells, stem cells are known for their ability to reproduce through numerous cycles of cell division, a process in which their genetic material, called DNA, is replicated. To test the stem-cell-like quality of these beta cells, researchers studied the frequency at which both normal and bottom beta cells underwent cell division. Their results indicated that bottom beta cells replicate DNA more frequently and thereby proliferate more than other beta cells. In addition, bottom beta cells exhibit several cell markers characteristic of stem cells. This finding suggests these cells actually express fewer qualities unique to beta cells, leaving the researchers to wonder whether this is the reason behind their ability to survive immune attack.
Duck and Cover
In addition to harnessing qualities of stem cells, researchers found bottom beta cells avoid immune attack by lowering their expression of surface markers that are marked as foreign by the body’s immune system. “In essence, they ‘duck and cover’ from the body’s own self-targeting immunological responses,” Herold said. This finding indicates that bottom beta cells are resistant to autoimmune attacks characteristic of Type I diabetes. “The biggest surprise is finding that the beta cells are not just waiting there helplessly to be recognized and killed; instead they can hide from immune attacks in order to survive,” said Joyce Rui, an associate scientist who contributed to the study.
Following the discovery that bottom beta cells can survive diabetes in mice, the researchers were interested in whether this subpopulation of beta cells was present in human cells. They cultured pancreatic cells from both Type I diabetic and healthy patients, and introduced foreign cells to mirror the immune stress that occurs in the body when the immune system targets its own cells. Their results indicated both types of cells—disease-infected and healthy, normal cell cultures—developed a subpopulation of beta cells, suggesting human cells produced these cells just as the diabetic mice did. “These changes may account for the chronicity of the disease and the long-term survival of beta cells in some patients,” Rui said.
Promising News
Together, Herold and his team have revealed a subpopulation of beta cells that arise during autoimmune attack preceding Type I diabetes. These cells show potential as breakthrough treatments for diabetics: it may be possible to someday revert these bottom beta cells back to normal beta cells to produce sufficient levels of insulin Type I patients lack. “We have evidence that the beta cell subpopulation occurs in beta cells in humans exposed to inflammatory mediators. But whether or not this occurs in humans with type 1 diabetes remains unknown,” Herold said. It still remains unclear the origin behind these bottom beta cells. Their research opens numerous pathways for future research, including whether this subpopulation of beta cells can be differentiated into functional beta cells, which would provide a major breakthrough for diabetic patients lacking these insulin-producing cells. It is a sweet discovery that may shift the way clinicians treat diabetes.
About the Author:
Jessica Trinh is a freshman and prospective biomedical engineering major in Branford College. She is the Vice President of Synapse and works in Dr. Jiangbing Zhou’s lab studying nanoparticle treatment for brain tumors as well as Dr. Kamil Detyniecki’s lab studying pediatric seizure
clusters.
The author would like to thank Dr. Kevan Herold and Dr. Jinxiu Rui for their time and enthusiasm for sharing their research.
Extra Reading
Herold, K.C., Vignali, D.A.A., Cooke, A., Bluestone, J. 2013. “Type 1 diabetes: translating mechanistic observations into effective clinical outcomes.” Nature Reviews Immunology 13, 243-256. doi: 10.1038/nri3422.