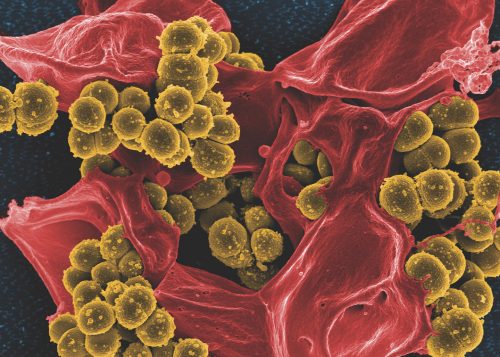
When threatened, bacterial cells come together to build a city. This city contains many multi-storied apartments. It has intersecting channels and canals that allow for materials to flow in and out. Yet, they are only made when bacteria, which are normally independent organisms, become socially motivated. When they are under stressful conditions, bacteria come together to build cities, trying to protect themselves from the stress. These cities are called biofilms, a highly organized community of bacterial cells, and they could be growing inside your cells. A research team at Yale, led by Andre Levchenko, built one of these cities to better understand how they are able to form.
A dangerous place
Biofilm infections are deadly killers. These cities made by bacterial cells can form on surgical equipment and infect patients—killing them during their recovery. Even with simple cosmetic surgeries, there is still a risk of clinical infections caused by biofilm formation. According to the National Institute of Health (NIH), 65 percent of bacterial infections and 80 percent of chronic infections are associated with biofilm formation. Biofilms can also contribute to antibiotic resistance, which weakens the ability of some medicines to treat bacterial infections. Biofilm formation is a dangerous threat for recovering patients. Though surgeries or other procedures may have solved one problem, a patient may still be at risk for developing a deadly biofilm infection. Yet strict procedures and guidelines are offered to recovering patients to prevent these infections from happening.
Biofilms are also prevalent in cystic fibrosis, a genetic disease affecting the lung cell environment in children and teenagers. Bacterial cells take advantage of and thrive in these mutated lung environments. They settle in this region and form biofilms, which create byproducts that clog the airway and can potentially kill the afflicted patient. Medically, biofilms are central to many problems, motivating several researchers, including Levchenko and his team, to better understand their formation.
A social life
When stressed, bacterial cells can sense the byproducts of metabolism. Researchers have found that when you build a maze with small rooms for bacterial cells, the cells will pack into the smallest room they can find. As a swarm forms, it will attract more bacterial cells as more byproducts are produced. It does not matter that, in this one small room, the cells are consuming the available food at a faster rate, because this congregation now has the power to do something as a larger unit. “They can count themselves, grow, and trigger new behaviors. They behave as a community instead of as individuals,” Levchenko said.
Because they grow so close together, these bacterial cells are also able to physically pass genetic material around by way of direct contact, a process called conjugation. As a result, collective resistance in a bacterial population emerges more quickly because genes associated with antibiotic resistance can be passed throughout the population. Bacterial cells can also actively communicate with each other. Known as quorum sensing, this process occurs when there are a sufficient number of cells in one place. “They are able to count themselves to see if there is a certain number to start doing something,” Levchenko said. The social environment created by this bacterial population may contribute to biofilm formation.
High-stress
To study biofilm formation, Levchenko and his team first built a contraption to grow the biofilm. “We decided to build what looks like a hotel for them with rooms and corridors carrying nutrients and waste,” Levchenko said. “Without these devices, you would never get to the biofilm formation stage because the cells would just escape.” Each room hosted a growing bacterial community enclosed on three sides by rigid boundaries with narrow chambers through which the researchers could flow through nutrients as needed.
However, the researchers wanted to assess the mechanical stresses of the bacterial colonies, and because the cells continued to break out of this device, they added an elastic roof to the chamber to allow for the added pressure of the growing biofilm colony. As cells reached the capacity of the chamber, they could continue growing by expanding the elastic roof. The researchers could measure pressure produced due to deformation of the roof. Newton’s third law states that for every action there is an equal and opposite reaction. “As the colony presses on the walls of the chamber, the chamber presses back on the colony, making them physically stressed,” Levchenko said. Thus, by confining the bacteria into physically stressful environments, the bacteria responded by growing and building up pressure in the chamber. The researchers wondered whether mechanical stress generated by growth was related to the formation of deadly biofilms.
Biofilm formation
In the body, antibiotics present a threat to the survival of bacteria. When faced with such threats, bacteria attempt to protect themselves. They often do this by packing themselves tightly in isolated niches in complex microenvironments. Other times, bacteria such as E. coli, seek shelter inside of host cells of the urinary tract and form populations called intracellular bacterial colonies (IBCs). In addition to providing nutrients for growth, the host cells offer protection from the host immune system. Using their chamber equipped with an elastic roof as a model for IBCs in cells lining the urinary tract, Levchenko and his team used E.coli to study biofilm formation.
The researchers tested for biofilm markers in the E. coli chambers as the elastic layer began to deform. Specifically, they fluorescently labeled two biofilm indicators, exopolysaccharides (EPSs) and curli fibers. EPSs play an important structural role in the development of biofilms. Curli fibers are extracellular structures which enhance the adherence of bacteria to the host cells. The researchers observed a substantial increase in the expression of both biofilm markers in the chambers with deformed chamber roofs. This confirmed that self-induced stress due to mechanical stretching of the elastic layer of the chamber led to biofilm formation.
Growth of E. coli in cells increases the stress within the cell. The researchers showed that this self-induced mechanical stress due to growth leads to biofilm formation. The consistency of a biofilm is viscous, like honey, which prevents biofilms from continuing to grow. Like stirring honey, the faster you stir, the greater the resistance you will face. The viscous environment of the biofilm induces more stress. More stress perpetuates the positive feedback loop and promotes more biofilm formation. Because biofilm development increases antibiotic tolerance, infections caused by biofilms are very dangerous.
Under pressure
Turgor pressure is the pressure exerted on the cell wall by the osmotic flow of water into the cell. However, similarly to how the bacteria exerted pressure on the elastic layer of the chamber, bacteria can also exert pressure on cell membranes. If the pressure due to bacterial growth exceeds the turgor pressure, the host cells can burst.
Levchenko and his team found that the pressure measured in their chambers at the point when the cells stopped growing was lower than the known turgor pressure. Because growth stopped at such a low pressure, this suggests that the E. coli colonies adapted to the stress of the chamber and ceased growth before the host cell membranes burst. As such, the mechanism for stopping growth was self-imposed by the stress made from biofilm formation. The ability of the E. coli to cease growth may be related to the viscous consistency of biofilms. “Growth may stop because of the increased viscosity of the cell environment caused by biofilm formation,” Levchenko said. Cells that grow more rapidly in this viscous environment therefore experience more stress and halt growth.
The future
Now that we know the mechanisms for cell survival under stressful conditions—controlling stress and biofilm formation—we have a better understanding for the consequences of biofilm formation and antibiotic tolerance during uropathogenic infections. “Now we can look at other bacterial biofilms,” Levchenko said. By understanding the bacterial cells’ adaptive response to stress—forming biofilms—we can learn more about fighting infections. Antibiotics induce stressful conditions for the bacterial cells, which leads to biofilm formation and increased antibiotic resistance. “You need to be careful stressing the cells. Sometimes the more you fight them, the worse they become because they will form a defense of biofilms,” Levchenko said. With a better understanding for the biofilm mechanism for antibiotic tolerance, researchers can begin to deal with any biofilm-related clinical or industrial problem.
With this information, researches can also begin to pursue anti-biofilm drugs. The chamber built by Levchenko’s team to observe biofilm formation provides a completely controlled method for testing different drugs. “You can add a compound that may become a drug to different rooms and now you can test thousands of compounds,” Levchenko said. Not only was the chamber useful in determining the method for biofilm formation, but it can also be practically used for screening potential drugs.