In 1993, scientists working for pharmaceutical giant Pfizer had a conundrum on their hands. Their new wonder drug, the product of years of intense and costly research, failed to show any effectiveness in treating patients with angina pectoris, a common cause of severe chest pain. Then named UK-92,480, the drug was destined to be shelved. if it were not for the keen observation that some patients reported sustained erections as a side effect of their treatment. The researchers were baffled at first but then realized the opportunity on their hands. Five years later, UK-92,480 gained FDA approval as an oral treatment for erectile dysfunction and was released to market. Today it has been rebranded as sildenafil — or Viagra — and has become a windfall for Pfizer.

Could other failed drugs find their own stories of serendipity? Certainly, finding novel uses for failed drugs is not a new idea. Aside from Viagra, a number of well-known drugs had originally been developed for other purposes, such as Rogaine, which had started as a drug for high blood pressure, and AZT, the anti-HIV drug that was originally supposed to be a cancer drug. In each case, advances in our understanding of diseases and human biology led researchers back to the past, repurposing old drugs based on a better understanding of their mechanisms of action.
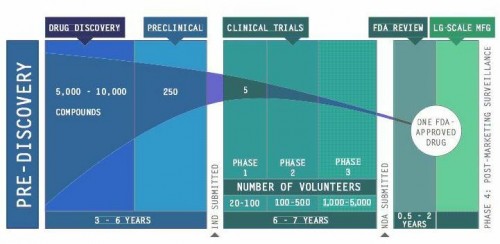
Pharmaceutical companies have taken an interest in reviving their failed drugs. From their perspective, drug development is a risky business. Bringing a drug from the lab to the clinic typically takes 13 years and an investment of around $1 billion, with a 95 percent risk of failure. Some drugs may not be structurally suitable for efficient mass production, some show dangerous side effects, and some simply do not work against the target disease. In total, around 30,000 drugs have been shelved by pharmaceutical companies over the past three decades, and some of these failed drugs have shown new promise for treating other diseases. Because they have already been tested in humans, details about their production, dosage, and toxicity are readily available, which can expedite the process of developing new disease treatments. Instead of starting from scratch, successful repurposing of even a few drugs could save companies substantial amounts of cost and time.
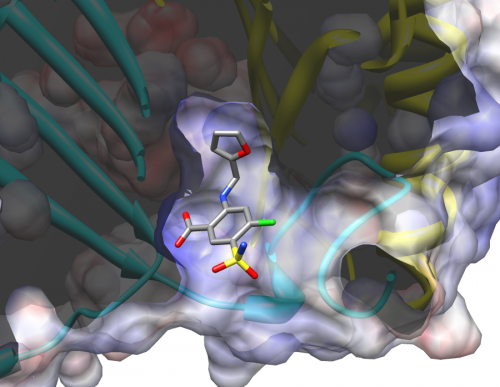
A new development in drug retargeting strategies has been the creation of drug libraries that allow receptor sites to be matched up with pre-existing chemical compounds. Last year, Dr. Elias Lolis, Professor of Pharmacology at Yale School of Medicine, and Dr. Michael Cappello, Professor of Pediatrics, Microbial Pathogenesis, and Public Health at Yale School of Medicine, jointly published a paper detailing how this approach can be applied to treating hookworm infestations. Previous research had suggested that hookworms manipulate the human immune system by mimicking a key human regulator with their own protein, AceMIF. Together, Lolis and Cappello’s research teams screened a chemical library of almost a thousand FDA-approved compounds for possible drugs that could inhibit AceMIF activity, effectively preventing a hookworm from shutting down the human’s immune response. From this study, they were able to identify two potential anti-hookworm drugs previously tested for other purposes: sodium meclofenamate, an anti-inflammatory drug, and furosemide, a diuretic.
Recently, the National Institute of Health, through their National Center for Advancing Translational Sciences (NCATS), launched a massive $20 million program to reopen research into 58 drugs shelved by various pharmaceutical companies. Worth up to $2 million each, these grants will be awarded to proposals from academics, non-profit groups, and biotechnology corporations investigating novel applications for these failed drugs. Even this effort, however, has not been without controversy. Some, like former Pfizer President John LaMattina, have criticized the NCATS undertaking, claiming that companies themselves have already taken similar rediscovery initiatives.
Others worry about potential intellectual property issues that may impede the process to push the repurposed drug through to the clinic. Companies may be hesitant to sacrifice any measure of intellectual property rights of their compounds, which are central to their value. On the other hand, without patent protection, researchers will have a difficult time convincing companies to continue developing off-patent drugs and bring them to market. Although advances in both biological understanding and computational technology offer exciting possibilities in old drugs, the road to the clinic remains long and treacherous.