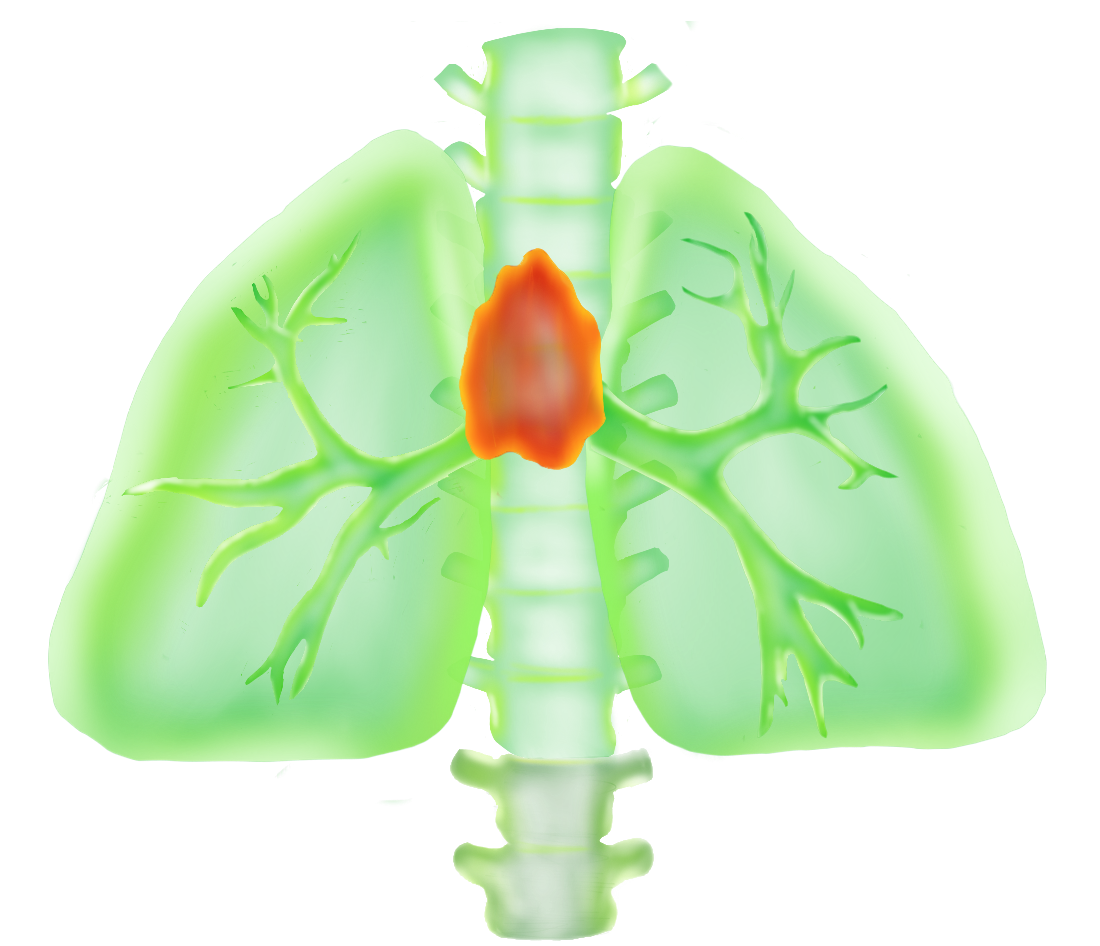
As we grow older, our immune systems begin to falter.
Colds hit harder. Responses to vaccines turn weaker. One of the primary culprits in the deterioration of immunity with age is the failure of the thymus, a tiny organ tucked between the heart and the breastbone that is vital in the production of disease-fighting T cells. With age, this organ becomes gorged with fat, compromising our ability to make new T cells. So when we are old and the winter sniffles hit, they hit hard.
Now, a study led by Vishwa Deep Dixit, professor of Immunobiology and Comparative Medicine at the Yale School of Medicine, has uncovered a hormone that may help curb thymic breakdown. Known as Fibroblast Growth Factor 21 (FGF21), this hormone may stimulate the thymus and prevent our immune systems from going downhill as we age. This finding provides new biological insight into the factors involved in thymic aging. It may also offer a promising treatment to boost immunity in the elderly and in cancer patients after bone marrow transplants.
A first clue
Most organs deteriorate with age, but the thymus is notable for being one of the first to go. The early collapse of the thymus is problematic because this small organ has an important function. The thymus nurses young T cells as they mature, providing them with a rich environment of signals to guide their development. The mature T cells then leave the thymus and go on to establish a vast population of powerful, highly specific immune warriors that are critical in protecting our bodies against infection.
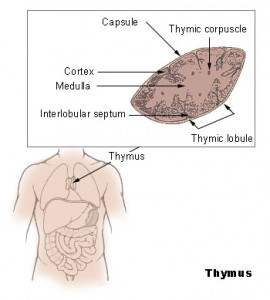
However, this healthy T cell nursery does not stick around for long. Instead, as we grow older, the thymus becomes packed with fat cells. By the age of 45, long before most other organs have shown any signs of aging, the thymus is over 70 percent fat, Dixit said. While it is not clear where these fat cells come from or why they are there to begin with, the damage they do is devastating. By the time we reach our mid-forties, when our T cells journey from the bone marrow where they are formed to their thymic nursery, they arrive to find it in shambles — jam-packed with fat cells, its architecture collapsed. Any hope of T cell maturation in this environment is practically nonexistent, and the release of new, mature T cells from the thymus grinds to a halt. This explains why older people have weaker immune systems. Yet why and how the thymus falls into disarray is still not fully understood.
A clue to the dynamics of thymic fatty deterioration came almost seven years ago, when Dixit and his colleagues were analyzing mice raised on a low-calorie diet. Calorie restriction promotes fatty acid breakdown to continue to fuel the body, which has long been linked to increased lifespan. When researchers looked at the thymi of these calorie-restricted mice, they noticed something surprising — the thymi were remarkably healthy. Unlike the thymi of mice fed on normal diets, the thymi of these mice had intact structures and were relatively free of fat. Curious, Dixit and his colleagues analyzed what proteins were being expressed at elevated levels in the thymi of calorie-restricted mice. The hormone FGF21 was one of them.
At the time, FGF21 was known primarily as a hormone secreted by the liver that promotes fatty acid degradation during times of energy deficit; it was also known to extend lifespan. Dixit wondered if this hormone could help mediate the beneficial effects of calorie restriction on thymic aging by promoting the breakdown of fatty acids, thus preventing the thymus from becoming crammed with fat and slowing the organ’s deterioration. “Our thinking was that if we could elevate FGF21 within the thymic micro-environment, it would prevent lipids from accumulating in the thymus and help maintain thymic architecture,” Dixit said. “We would essentially be mimicking calorie restriction.”
Good as new
Armed with this promising initial finding, Dixit and his colleagues first set out to determine where, when, and how much FGF21 is expressed in the thymus. Intriguingly, it turns out that the expression of this hormone in the thymus steadily drops with age. It also turned out that just 1% of cells in the thymus — a population of cells called thymic epithelial cells (TECs) — are responsible for both producing and responding to this hormone. The thymus is mainly populated by immune cells, but it is the TECs that are key players in guiding the development of T cells. “The fact that FGF21 was coming from and acting on such an important cell type for thymic function suggests that this molecule must be really important in the thymic environment,” Dixit said.
The researchers took the logical next step. They decided to investigate what would happen if they ramped up expression of FGF21. They aged mice that were genetically modified to express high levels of this hormone and then took a look at their thymi. What they found was exciting: the thymi of elderly mice that were engineered to overexpress the hormone looked very similar to those from the calorie-restricted mice. Compared with mice with normal levels of the hormone, these mice had fewer fat cells, more T cells and certain TECs, and more intact thymic architecture. “The thymus of a one-year-old mouse that overexpressed FGF21 looked like the thymus of a four-month old mouse with normal FGF21,” Dixit said.
In the mice with more FGF21, a healthier thymus meant a more robust immune system. The researchers found that the mice with more of the hormone were producing more new T cells. By slowing the deterioration of the thymus, FGF21 protected the mice from immune system collapse that comes with age. On the flip side, the researchers found that in mice in which the FGF21 gene was knocked out, thymic degradation was accelerated, and the number of new T cells produced was limited. Without this hormone, the thymus collapses too soon.
An unknown mechanism
While the power of FGF21 in preventing thymic degradation is clear, its exact mechanism is still unknown. One potential explanation, which is in line with its previously established metabolic functions, is that this hormone promotes breakdown of fatty acids in the thymus. “This could prevent the thymic stroma from becoming infiltrated with lipids and help maintain the microenvironment so that the thymus can continue making T cells,” Dixit said. While this explanation seems plausible, it has not yet been demonstrated.
Dixit thinks FGF21’s role in promoting fat breakdown might not be the whole story. His suspicion stems from the surprising fact that this signal is produced in the thymus at full blast in the young, even though there is very little fat in the thymus at this point in time. This suggests that the hormone might have another function in addition to fat clearance that acts early on. Dixit speculates that FGF21 may have an additional role in triggering signaling pathways in TECs associated with cell division, thus allowing these crucial epithelial cells to proliferate and maintain themselves. If this proves true, it would mean that what scientists have long considered to be solely a metabolic hormone actually has another crucial function. Evolutionarily, this possibility raises interesting questions about which came first, FGF21’s function in metabolism or in cell growth.
With these issues still up in the air, Dixit said the next step is to investigate the hormone’s molecular mechanism. His group is currently working on dampening or elevating FGF21 levels in just the thymi of mice to eliminate its potential effects on other parts of the body, and then homing in on exactly how this hormone regulates thymic epithelial cells.
Therapeutic promise
Though its mechanism is still being clarified, the ability of FGF21 to slow thymic deterioration is clear, and could be harnessed therapeutically. Drugs that raise its levels could be used to improve thymic function and enhance T cell production in the elderly, offering hope that immunity can be boosted in older individuals.
Additionally, this hormone holds promise in treating cancer patients who have undergone bone marrow transplants. Prior to bone marrow transplants, a patient’s old, damaged set of blood and immune cells is wiped out with chemotherapy. Then, their unhealthy bone marrow is replaced with fresh, healthy bone marrow, which is used to repopulate their blood and immune cells. But if the patient is over the age of 45, the new T cells generated from the transplanted bone marrow will arrive at the thymus for maturation only to find it old and packed with fat, meaning that the patient is unable to produce any new, mature T cells.
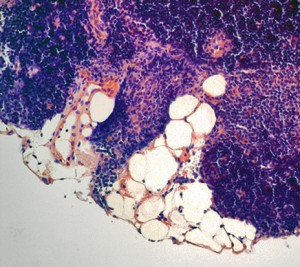
Since their original T cells would have been wiped out by chemotherapy, the patients are left without any T cells, rendering them extremely susceptible to infection. To make matters worse, the few original, unhealthy T cells that were somehow able to survive chemotherapy can take hold and start proliferating in the patient’s body. According to Dixit, this nightmare situation could be remedied by treatment with FGF21 — by rescuing thymic function, this hormone could allow these patients to start replenishing their T cell populations.
Yet more research is needed before this hormone can be administered as a drug to improve thymic function. Moving beyond experiments conducted using mice genetically engineered to overexpress the hormone, Dixit’s lab is now attempting to deliver the hormone in the form of a drug. A major challenge is figuring out how to maintain elevated levels of the hormone for a substantial period of time, a challenge compounded by the hormone’s short half-life.
Despite these challenges, FGF21 therapy appears to be a promising approach that could have substantial benefits, and Dixit and his team is working to turn it into an effective therapy. If they are successful, aging immune systems in need of rescue could — in the not-so-distant future — be bailed out by a tiny, life-giving hormone.
Extra Reading:
Yang, H et al. (2009) Inhibition of thymic apidogenesis by caloric restriction is coupled with reduction in age-related thymic involution. J Immunol 183 (5): 3040-3052.
About the Author:
Malini Gandhi is a junior in Morse College majoring in Molecular, Cellular, and Developmental Biology. She is interested in immunology, microbiology, and evolutionary medicine.
Acknowledgements:
The author would like to thank Dr. Dixit for his time and enthusiasm in discussing his work.