Think that cancer can only be treated by means of dangerous chemotherapy, radiation, or a foreign substance created in test tubes? Think again. Researchers at the Yale Cancer Center and the Yale School of Medicine believe that a certain type of cell in your body could provide remarkable new insights on the treatment of cancer.
Researchers from Yale School of Medicine professor Madhav Dhodapkar’s lab at the Yale Cancer Center investigated memory T cells in patients with melanoma — a type of skin cancer. This research, which was led by first authors Chandra Sekhar Boddupalli, Noffar Bar, and Krishna Kadaveru, was published in the Journal of Clinical Investigation in December 2016. The team embarked on an investigation of drugs that target the special checkpoints in the immune system. Previous studies had suggested that only a small subset of T cells — special cells that attack foreign invaders in the body — express these inhibitory checkpoints within tumors. To improve the success rates of these treatments in patients with melanoma, the researchers started studying the T cells in the tumors of their patients, aiming to characterize function and properties of these cells to improve immunotherapy drugs.
New Section Heading Here?
The researchers studied T cells, which allow a host organism to have a quicker immune response against a pathogen to which the host’s immune system has already been exposed. T cells are not extremely rare or aberrant, nor are they specific to cancer patients. Rather, they are a part of a normal human immune system. In fact, if you have ever had an infection or disease — even a simple rash on your skin — then these cells are probably working as part of your immune system today. The immune system’s job is to help preserve the internal conditions necessary for life processes by combating potential threats from foreign agents. On any given day, our bodies are exposed to a myriad of these threats — malicious bacteria on the door handle we touch, viruses we are exposed to by our neighbors, deadly tumors we develop, or even toxic chemicals in the air that we inhale. Such substances or organisms that can cause diseases are called pathogens, which are specifically targeted, often destroyed, and remembered by a variety of specialized cells utilized by the immune system.

Each T cell has a specific receptor that can only bind to a specific antigen, the part of a substance or organism that a T cell can identify. Upon binding to the antigen, T cells can recruit other immune cells to help eliminate the foreign agent. The Yale researchers studied a special class of T cells called memory T cells. Memory T cells are unique because they persist in the body after the threat has been removed. Moreover, if the host’s memory T cells are re-exposed to that specific pathogen, the cells quickly replicate to magnify and facilitate the immune response to the threat. Vaccines, for example, take advantage of this mechanism to create memory T cells for pathogens, so that a faster immune response occurs when people are exposed to these pathogens a second time, helping the person recover more quickly.
Although most T cells recognize foreign agents as pathogens, every person has a certain number of autoreactive T cells, which trigger an immune response to the person’s own cells. When T cells are too active, the rate of autoimmune response increases. To prevent over-reactive T cells and their autoimmune consequences, the immune system has inhibitory immune checkpoints — built-in mechanisms that keep T cells from becoming too active. These inhibitory checkpoint molecules can also be expressed by cancer cells, which helps to explain why cancer is so deadly — it represses immune response that normally could remove the diseased cells. Recently, immunotherapy treatments for cancer have been developed in which certain drugs remove the inhibitory effect from T cells, allowing them to fight against the tumor. However, these anti-inhibitory drugs only work for a small percentage of melanoma cases, leading the researchers to further investigate ways to improve treatments, especially through the use of memory T cells.
It was once believed that memory T cells were in constant circulation in the blood and that as they circulated throughout the body, they would arrive at the tissues requiring an immune response. However, in the past few years, scientists have found that while some memory T cells that travel in the blood, certain types of memory T cells travel to an infected site within a tissue and then stay at that site for an extended period of time. Because these special memory T cells don’t circulate throughout the body, they are ready to rapidly generate an immune response when the same pathogen enters the tissue again. Such memory T cells are called tissue resident memory T cells (TRM cells), since they reside in the tissues.
While studying the T cells within melanoma tumors, the Yale researchers realized that the T cells that express the inhibitory checkpoints have the markers of tissue resident memory T cells. They also found that these T cells express genes previously known to be expressed by tissue resident memory T cells and that that there were differences in the T cells that were present in different tumors within the same patient. Recent clinical studies with immune checkpoint blocking agents have shown that occasionally, some of the tumors in a patient responded to treatment, while other tumors in the same patient did not respond. The researchers hypothesized that the discrepancy in the responses in such cases may be due to differences among T cells in different tumors, and these differences persist because these T cells were not in circulation and could not travel to other tumors, a characteristic of TRM cells. To ensure that the cause of the differences in the tumors’ responses was the TRM cells and not the nature of the tumors themselves, the team ran tests to compare the expression of the tumors’ protein-coding genes. They found fewer differences in protein expression existed between the different tumors than between the different T cells found in the tumors. From further research conducted after the paper was published, the group hypothesized that the differences among T cells and not the tumors were the factors causing the different responses. Further supporting their claim that the tumors’ T cells were TRM cells, the researchers found a specific type of transporter and markers of TRM cells in the tumor cells.
A New Hope with Adaptive Therapies
The ability of TRM cells to protect against pathogens and potentially protect against tumors makes them especially enticing for more scientists to research. Still, even though there are proposed transporters and markers for TRM cells, much remains unknown about these cells. “We need to pay attention to understanding their function, what they are, what they are recognizing, because that will help us treat these patients,” said Dhodapkar.
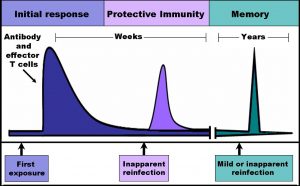
“There is a very urgent need to understand the functional biology of these tissue resident memory T cells,” Boddupalli said. “It is very important to understand these tissue resident memory T cells because it can give us better perspective in designing targeted therapies for autoimmune diseases, and also for cancer.”
Cancer has long seemed like an incurable disease. Each tumor is so different that it is hard to imagine a single unified cure. For example, existing cancer vaccines — in which patients are exposed to many cancer antigens with the aim of triggering a strong immune response — are not very effective. Thus, it is no surprise that T cell therapies for most diseases generally only work on a small percentage of people However, the reason that only some people respond to therapy is not well elucidated, partially because the functions and properties of the T cells used are poorly understood.
“We give millions of cells for the adoptive T cell therapy, but very few of [the T cells] actually get into the tumor and stay there. So we need to learn how to make that happen,” said Dhodapkar. To fully achieve an understanding of the functions, mechanisms, and needs of these cells, scientists can utilize TRM cells as a tool that can arrive at the site of a tumor, stay there for a long time, and eliminate the cancer. The researchers’ work has been a big step in fully understanding these TRM cells and, by extension, creating a more successful cancer immunotherapy.
Today, a myriad of new discoveries, techniques, and possible cures to disease is emerging, making it seem feasible to solve problems once deemed unsolvable. Perhaps a more universal cure for cancer is on its way!
About the Author: Diyu Pearce-Fisher is a sophomore Biomedical Engineering major in Berkeley College. She works in Dr. Stuart Campbell’s lab.
Acknowledgements: The author would like to thank Dr. Kavita Dhodakpar and Dr. Sekhar Boddupalli for the time and efforts towards facilitating the process of writing this article.
Extra Reading: Bindea G, Mlecnik B, Angell HK, Galon J., 2014. “The immune landscape of human tumors: Implications for cancer immunotherapy.” Oncoimmunology. 3(1). doi: 10.4161/onci.27456