Imagine two college students; let’s call them John and Jack. They are both in an especially hard lecture class with a not-so-forgiving professor. They spend days preparing for their midterm to no avail—both do poorly. From the outside, John and Jack are both generally happy people. But, while John picks himself back up and works harder for the next exam, Jack falls victim to depression. So what’s the difference between John and Jack? Most would assume that Jack is mentally weaker—he just gave up. Perhaps it’s more than just psychological; what if Jack was born more vulnerable to stress?
Past studies have demonstrated how exposure of drugs, radiation, or poisons in the womb, or prenatal exposure, can damage cells. These children tend to be more vulnerable to mental disorders associated with cellular stress, as if these disorders have been pre-programmed during early development.
So, looking at a group of people or animals, how can one tell who is vulnerable? A team of researchers at Yale, led by Professor of Neuroscience Pasko Rakic, has developed a way to identify and visualize these at-risk cells using fluorescent markers. This new identification system may lead to further advancements in the research of the development and treatment of mental disorders, such as epilepsy, autism, and schizophrenia, some of which are caused by prenatal exposure to environmental stress.
A cell’s response to stress
Heat shock factor 1 (HSF1) is a part of a signaling pathway induced by cellular stress. Heat shock factors are transcriptional activators, which means they bind to DNA at specific locations to turn genes on and off, regulating the quantity of heat shock proteins produced. Heat shock factors are key in analyzing a cell’s response to stress; therefore, by studying heat shock factors, scientists aim to learn more about how mental disorders may develop in response to stress.
In earlier studies by other scientists, cells were grown in tissue cultures and divided into groups that were exposed to heat and normal controls. Researchers found that many cells exposed to heat generated a much higher level of one specific protein, dubbed heat shock protein (HSP), before they died. The surviving cells had increased levels of heat shock factor, which served as their protector. “It’s not just that one could be exposed to too much heat—a lot of people are confused by the name heat shock factor. However, the same cell reaction could occur after overexposure to anything; you could have too many x-rays; you could drink too much gin; you could eat too much mercury from fish,” Rakic said.
Rakic and his colleagues observed that when pregnant mice were exposed to harmful agents such as alcohol, x-rays, and methyl mercury, some fetuses died while others appeared normal. “The fetuses that survived had developed just the right amount of heat-shock factor that prevents their death,” Rakic explained. “When the cells survive, they look normal, but when you expose those animals a second time to harmful conditions postnatally, they are more vulnerable to brain disorders.” The same is true in humans—people who are exposed prenatally to drugs could appear normal, yet be more vulnerable when exposed to stress after birth.
Glow up before growing up
Because they observed a relationship between the level of HSF1 and the development of mental disorders after a second exposure to harmful elements or stress, the research team used the presence of HSF1 to identify vulnerable brain cells that may contribute to the development of disorders. A specific sequence of DNA that produces Red Fluorescent Protein (RFP) was inserted into the mouse DNA next to the gene that coded for heat-shock factor. This insertion results in a protein that emits a bright red fluorescent “glow” when exposed to high-energy light—moreover, the fluorescence would only be present in cells producing the heat-shock factor. Thus, this technique enables the identification of the more vulnerable cells that produce stress-induced heat shock factor.
“What we did is attach red fluorescent protein, so that the cells with heat-shock factor appear red under the microscope. That is why it is called a reporter system,” Rakic said. This system was tested by introducing the gene for the fluorescent protein into mice and analyzing the amount of red fluorescence under various conditions, such as the absence of heat shock factor and the mutation of the reporter system DNA.
Mice that didn’t have the HSF1 didn’t display red fluorescence, confirming the reliability and specificity of the reporter system. The scientists determined that the reporter system specifically detects the presence of the heat shock factor and thus can be used to label cells vulnerable to stress with a bright red color.
After the reporter system was validated, the researchers developed live mice and cell cultures with the red fluorescent reporter DNA inserted. The use of mice was key to the possible implications of their results, as mice are model organisms that can give insight into how biological processes occur in humans. “This reporter system may provide a powerful tool for exploring the pathogenesis and treatment of multiple disorders caused by exposure to environmental stress before symptoms become manifested, exacerbated, and/or irreversible,” said Masaaki Torii, Visiting Assistant Professor of Neuroscience at Yale and Principal Investigator at The Children’s Research Institute.
Finding the odd ones out
Two methods were used to detect and characterize vulnerable neurons: first, experiments were conducted in petri dishes with reporter brain cells in order to analyze the behavior of the vulnerable cells compared to normal cells when exposed to stress. Second, experiments were conducted with the live reporter mice to analyze the effects of stress on vulnerable brain cells.
Preliminary experiments with reporter cell cultures were performed under various concentrations of alcohol (to simulate alcohol intake during pregnancy) and various temperatures. Rakic and his colleagues observed that higher concentrations of alcohol, higher temperatures, and longer durations of heat corresponded to greater red fluorescence in the cells. This indicated that, as the amount of physical or chemical stress placed on the cells increased, the amount of cellular stress response also increased.
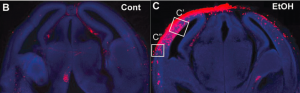
Later experiments in mice models involved exposing mice to alcohol and other environmental stressors including hyperglycemia (an excess of sugar in the blood stream) and asphyxia (oxygen deprivation) during prenatal development. Post-birth, these mice had increased red fluorescence in the cortex, the frontal, outer layer of the brain, suggesting that these red cells had an activated stress response and increased levels of HSF1. “We sacrificed the mouse and saw in slices of brain tissue that some neurons had a red color due to the reporter. These neurons otherwise looked normal, but we knew that they were vulnerable due to the red labeled HSF1,” Rakic said.
The team of researchers also saw red fluorescence in damaged cells in other organs of alcohol-treated mice, suggesting that the reporter system can be used in other tissues and organ systems, a finding that may be important to future studies of other diseases that develop due to exposure to harmful agents during early development. “We can now study… how and why som people react more strongly to stress,” Rakic said.
Diagnosing the red cells
Finally, and most importantly, the researchers observed that these vulnerable neuronal cells were different structurally and behaviorally than normal neurons. “The trickiest part was to confirm that the cells identified by the reporter really show abnormal physiological properties. We addressed this by analyzing the physical cell forms, and cell migratory behavior and electrical properties,” Torii said.
When exposed to alcohol and heat, red fluorescent neurons with the increased HSF1 were observed to have shorter neuronal signaling branches, called axons, which are responsible for conducting electrical signals from neuron to neuron. Additionally, in mice exposed to alcohol during early development, the vulnerable cells were observed to move more slowly than the surrounding normal cells. This not only shows that the reporter system successfully differentiates between damaged and normal cells, it also suggests that damaged neurons behave differently due to the environmental stress faced during early stages of brain development, which may have implications in further studies of mental disorders.
The reporter system developed by Rakic, Torii, and the rest of the team serves as a window into the cellular basis of mental disorders. “Some cells are vulnerable. They are more sensitive; and it’s not just to one thing. They may have increased sensitivity to stressors like losing a job, or exposure to alcohol or drugs. They are just not quite as resistant,” Rakic said.
This new ability to distinguish vulnerable cells from normal cells has implications for the identification of individuals who are more susceptible to mental disorders. Children born to mothers who were using drugs or alcohol during pregnancy are likely more vulnerable to developing disorders later in life. The ability to physiologically identify this increased risk is key in the treatment and study of these disorders. “Identifying damaged cells before serious symptoms arise is similar to finding small cracks in a wall or foundation of your house, which can cause serious destruction. The earlier you fix such small cracks before they can grow, the easier and better the repairs will be. The new reporter system helps early finding of such small cracks in humans,” Torii said.
Featured Image Art Credit: Sonia Ruiz
About the Author
Eileen Norris is a freshman prospective Biomedical Engineering Major in Ezra Stiles College. She is the production manager for the Yale Scientific Magazine and works in Professor Kavathas’ lab studying neoantigen-specific T cell responses in NSCLC patients undergoing immunotherapy.
Acknowledgements
The author would like to thank Dr. Rakic and Dr. Torii for their time and their enthusiasm about sharing their research.
Further Reading
Torii, M., Sasaki, M., Chang, Y., Ishii, S., Waxman, S. G., Kocsis, J. D., . . . Hashimoto-Torii, K. (2017). Detection of vulnerable neurons damaged by environmental insults in utero. Proceedings of the National Academy of Sciences, 114(9), 2367-2372. doi:10.1073/pnas.1620641114

