Imagine a production of all 38 of Shakespeare’s plays, performed back-to-back, complete with over one thousand characters and nearly a million words. The success of the production depends critically on each character saying the exact right words at the exact right times. Although the flawless execution of such a play seems daunting, the human brain orchestrates a similar task in the formation of 100 trillion synapses between over 100 billion neurons during development.
Synapses are the connections neurons use to communicate with one another, and the proper creation and maintenance of these connections are necessary for normal brain function. Exactly how the brain manages such complexity has been an interest of Professor Daniel Colón-Ramos of the Yale School of Medicine for nearly a decade. As we grow, our brains increase nearly fourfold in volume. The mechanism behind the maintenance of synapses during such dramatic growth is one of his lab’s primary interests. Current research in his lab has now uncovered that the regulation of a cell type called glia during growth may hold the key to synapse regulation.

C. elegans as a Model Organism
Due to the overwhelming complexity of working with the human brain, Colón-Ramos studies synaptic connections in Caenorhabditis elegans (C. elegans), a small roundworm about one millimeter in size. C. elegans is well-suited for neurodevelopmental research in several ways. One major advantage of C. elegans is the ease of genetic manipulation in the speices. Scientists in recent decades have also constructed the entire C. elegans connectome, which means that we now know the identity, morphology, and connectivity of each of the 302 individual neurons in C. elegans. Colón-Ramos described working with an organism with a completed connectome as “a bit like having an answer key. We know this is what a proper nervous system looks like so it’s much easier to notice when things go wrong.”
Discovery of cima-1
The vast majority of synaptic connections form while organisms are in their embryonic stages, and these connections remain as animals grow into adulthood. To address the question of how C. elegans maintains embryonically specified synapses into adulthood, the Colón-Ramos lab searched for genes involved in synapse maintenance through a process known as the forward genetic approach.
In the forward genetic approach, random mutations are chemically induced in the DNA of the organism, creating C. elegans with a wide array of abnormal features. These mutant organisms are then screened for particular characteristics of interest. To target genes involved in synapse maintenance, the Colón-Ramos lab isolated mutants exhibiting normal synapses as larvae but misaligned synapses as adults.

In order to uncover the exact genes and proteins malfunctioning in the mutants, the lab conducted genetic fingerprinting on the mutants’ DNA, a process similar to paternity tests in humans. The test pointed to a mutation in a gene called cima-1 as the cause of the abnormal synaptic connections. Prior to this study, cima-1 had never been investigated in C. elegans.
To further confirm that cima-1 acts critically during growth, the lab created mutations in the cima-1 genes of abnormally small and large worms. “It was a really fun experiment,” Colón-Ramos noted. “We had these really giant worms and really tiny worms, and exactly as we predicted, the small worms were less affected by cima-1 mutations and the giant worms were much more affected. It’s fun when science works like that.”

cima-1 and Glia
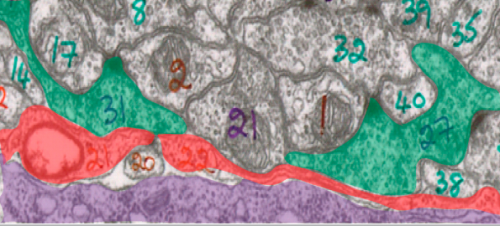
Because mutations in cima-1 cause abnormal neuronal connections, the logical prediction would be that cima-1 acts in neurons. Surprisingly, the Colón-Ramos Lab found that cima-1 was found not in neurons but in epidermal cells, which make up the outermost layer of the skin. So how does a gene found only in epidermal cells affect synaptic connections between neurons? The answer as it turns out, lies in glial cells.
Glia often act as translators between epidermal cells and neurons. Although previously believed to be nothing more than the “glue cells” of the brain, we now know that glial cells have a wide array of critically important functions including supplying nutrients, providing structural support, and fighting pathogens. Furthermore, recent research has implicated glial cells in a variety of neurodevelopmental activities, including synapse formation. In the case of neurodevelopment, epidermal cells help position the glia, and the glia in turn help position neurons and synapses. cima-1 mutants exhibited glial cells with abnormal shape, suggesting that mutations in cima-1 interfere with proper communication between epidermal cells and glia, resulting in abnormal synaptic growth.
An analysis of the various molecules involved in sending signals between epidermal and glial cells identified a receptor regulated by cima-1, suggesting that communication through this receptor was the mechanism behind synaptic maintenance. cima-1 was also found to regulate the number of these receptors that the body makes.
From C. elegans to Humans
Although humans and round worms seem to share very little in common with each other, evolution has ensured that even the most disparate of species share similar roots and, consequently, genes. The human equivalent of cima-1 is called sialin, and mutations in sialin result in a neurological condtion known as Salla disease. The defective sialin in Salla patients results in a dangerous build-up of a metabolic byproduct called sialic acid. Build-up of sialic acid causes brain atrophy and leads to an array of neurological problems including mental retardation. Patients also often suffer from motor control problems such as reduced muscle strength, loss of muscle coordination, and speech difficulties.
There is currently no cure for Salla disease, and symptoms can be managed only to a limited extent through medications and therapy. Although Salla disease is relatively rare, mutations in genes related to sialin cause other human diseases such as gout and congenital deafness. The Colón-Ramos lab hopes that further investigation of cima-1 and its related mechanisms will result in the discovery of novel therapeutic targets.
In addition to helping us better understand various diseases, the discovery of glia signaling may give us insight into the evolutionary origins of the human brain. Colón-Ramos pointed out that the epidermal-glia-neuron signaling network is reminiscent of the human blood-brain barrier. During times of high activity, neurons need more energy. Neurons communicate this energy need through glial cells that are in contact with epithelial cells of blood capillaries. The result is increased blood flow to the brain. The presence of the same pattern of signaling in C. elegans as well as humans suggests that the epidermal-glia-neuron signaling unit may be an evolutionarily ancient one.
Future Directions
Although the discovery of cima-1 and its role in neurodevelopment is a big step in understanding synapse regulation, Colón-Ramos noted that there are still many gaps left to be filled. For example, this research project only addressed how epidermal cells communicate with glia, and Colón-Ramos would like to further investigate how the glia communicate with neurons.
The implication that C. elegans and humans may share the same fundamental glial-neuron unit also raises questions regarding the evolutionary roots as well as functions of glia. Although not all of these questions are ones Colón-Ramos plans on pursuing, he hopes that “our research will influence the way other people and labs think about these questions in neurodevelopment.”
About the Author:
Somin Lee is a junior in Branford College majoring in Molecular, Cellular, and Developmental Biology. She currently works in Professor Marvin Chun’s lab investigating neural representations of sustained attention.
Acknowledgements:
The author would like to thank Dr. Colon-Ramos for his time and insights, and John Urwin for his clear explanations of complex topics.
