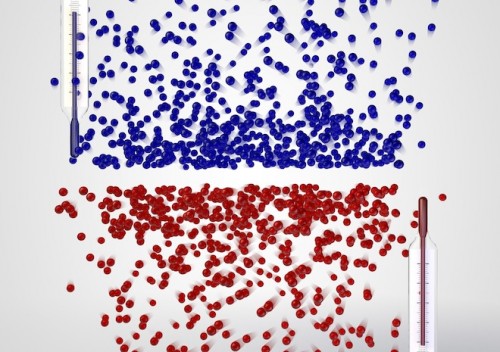
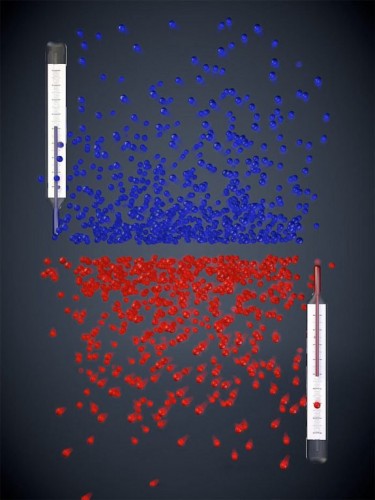
In high school chemistry class, we learned that absolute zero is exactly what its name suggests: the lowest possible temperature that can occur, the threshold at which atoms lose all their kinetic energy and stop moving. However, physicists have actually recently created an atomic gas that exists below this threshold, or at negative temperatures.
The term “negative” is actually a bit misleading. “The gas is not colder than zero Kelvin, but hotter,” explained Dr. Ulrich Schneider, the physicist in charge of the project. “It is even hotter than at any positive temperature — the temperature scale simply does not end at infinity, but jumps to negative values instead.”
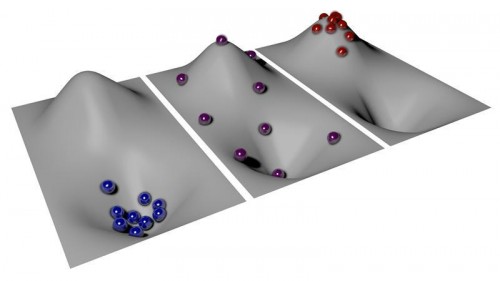
To explain the idea of negative temperature, the researchers describe their system in terms of hills and valleys. At absolute zero, atoms have no energy, and they are all at the bottom of the valley. As temperatures increase, some particles gain enough energy to move up the hill, but most remain at the bottom.

A temperature of exactly infinity is the balancing point. Here, enough particles have left the valley and spread out evenly along the hill’s slope. But past infinity, more particles are on the hill than in the valley — the exact opposite of the distribution in the positive temperature realm; this is what physicists call negative temperature.
In their groundbreaking experiment, researchers forced a gas into its highest possible energy state, achieving a temperature of a few billionths of a Kelvin below absolute zero. In the future, their work can allow for the study of other high-energy-systems that would otherwise collapse.
