We all owe much to the humble chloroplast. These tiny, solar-powered organelles, found only in plants, are responsible for a process called photosynthesis. Photosynthesis converts carbon dioxide into sugary fuel, and creates the oxygen we breathe as a byproduct. Yet chloroplasts have even greater potential. By using genetic engineering to “hijack” photosynthesis, a team of scientists hopes to transform them into biological factories. Alongside oxygen and sugar, their tweaked chloroplasts will produce other organic chemicals — ones we can use in medicine and industry.
This year, Poul Erik Jensen and colleagues published the results of their first chloroplast-modifying experiment. They successfully stimulated these organelles to produce dhurrin, a chemical used by plants to defend against herbivores. Dhurrin is not medicinally useful, but its successful synthesis in chloroplasts is an important breakthrough. Other plant-produced chemicals, including medicinal ones, may not be far behind. Using photosynthesis, future experimenters may “convince” chloroplasts to produce many products that are useful to humans.

Image courtesy of Molecular Expressions
Photosynthesis takes place in two parts, the “light reactions” and the “dark reactions.” The light reactions come first, and are fuelled by the power of the sun. When light rays hit the leaves of a plant, their energy is absorbed by chloroplasts and used to rip electrons from water. These electrons are high in energy, and can be used as fuel for other chemical reactions. Transfer molecules carry these electrons deeper within the chloroplasts, where they are used to power the dark reactions.
The purpose of the dark reactions is to convert carbon dioxide into sugar, which plants use as a source of food. But sugar production is a multi-step process, and creates many unintentional byproducts. One of these byproducts is oxygen, and another is the amino acid tyrosine. Tyrosine has no use to the chloroplast, and leaves the organelle after being synthesized. But in other parts of the cell, enzymes will convert some of this tyrosine into dhurrin. In short, photosynthesis naturally causes the production of dhurrin, but only in very small amounts, and not in the chloroplast itself.
Jensen’s team aimed to modify this system, driving the chloroplast to make dhurrin on its own. To do so, they had to relocate the cell’s dhurrin-producing enzymes, moving them into the chloroplast. If these enzymes were located in the chloroplast rather than the cytoplasm, they would encounter tyrosine as soon as it was produced. The enzymes would then immediately convert that tyrosine into dhurrin.
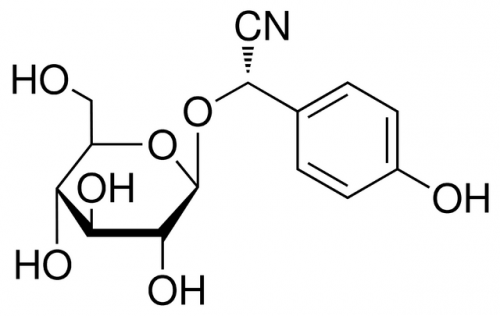
The enzymes could be moved, it turns out, by modifying their DNA blueprints. Enzymes, like all proteins, are built according to strict instructions. These instructions are written in the “language” of the genetic code and are stored in a molecule called DNA. If a molecule’s DNA instructions are changed, the cell will build it differently — or in this case, transport it to a different location. Jensen’s team modified the code for each dhurrin-producing enzyme, changing the “targeting sequence” that specifies the enzyme’s destination.
If all went as planned, Jensen’s genetically-engineered cells would send their dhurrin-making enzymes to the chloroplasts. There, the enzymes would encounter tyrosine, transforming it into dhurrin on the spot. The whole process would be powered by photosynthesis — as soon as the genetically-engineered plants were exposed to light, they would start making large amounts of dhurrin.
To test whether their modifications had succeeded, the scientists exposed their genetically-engineered plants to light. After allowing photosynthesis to proceed, they ground the plants up and tested their chloroplasts for the presence of dhurrin. Their positive results proved that the experiment worked. The chloroplasts of Jensen’s plants were making dhurrin on their own, and in much higher quantities than usual.
Jensen’s team succeeded in moving an entire biological pathway for the synthesis of a specific chemical from the cytoplasm into the chloroplast. Only small modifications were required for the genes involved, and the team is hopeful that other processes could be similarly relocated. Genetically-engineered plants may soon be churning out pharmaceuticals, powered only by the light of the sun.
Cover Image: The leaves of a plant absorb light, which will be used to power photosynthesis. Courtesy of Wallpapers Craft.
