
The blacklegged tick, Ixodes scapularis, is about two millimeters long and one millimeter wide in the nymphal stage of its life. Unless it is moving around on the skin, it can easily be mistaken as a dark brown freckle or speck of dust, despite its eight limbs. Many people never even see the parasite as it feeds on them for at least 36 hours; at that point, the tick has already transmitted the bacteria that cause Lyme disease.
The gut of this arthropod, just like the human gut, contains bacteria that benefit from their host without harming or helping it. These are known as “commensal bacteria.” In contrast, some of the commensalism in the human gut is actually mutualism, in which both host and parasite benefit; for example, E. coli bacteria in the gut help break down materials that humans cannot digest on their own, like cellulose.
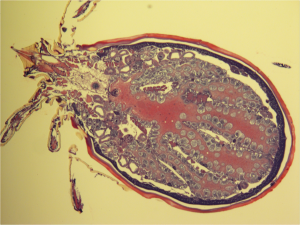
A Tick’s Bacterial Life Cycle
Ixodes scapularis has roughly a two-year life cycle, during which it progresses through separate stages: larva, nymph, and adult. Before molting and proceeding from one stage to the next, the tick must feed on a host to become engorged with blood. As it feeds, compounds in its saliva prevent the host from feeling the attached tick. Each of these feeding events is an opportunity for the tick to ingest bacteria from its host.
The bacterial inhabitant of the tick’s gut directly responsible for the spread of Lyme disease is a non-commensal spirochete called Borrelia burgdorferi, named after Willy Burgdorfer, who isolated and cultured the bacteria from the midguts of Ixodes scapularis ticks. Shaped like a helix, Borrelia is the etiological agent of Lyme disease. During a blood-meal, Borrelia travels from the blood and colonizes the midgut. Then, it enters the salivary glands where it can be secreted at a subsequent feeding event.
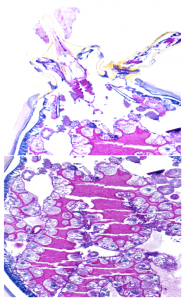
Alexia Belperron is a researcher in the Rheumatology Department at the Yale School of Medicine. One of her projects is studying Borrelia in infected mice using optical tweezers, or lasers. She warned that Borrelia is not to be underestimated, especially when it comes to motility. “It has to move within the [human] body and can get to every tissue within the body,” she said. “It moves into your bloodstream and then moves into tissue.”
To travel to other parts of the human body, Belperron said, Borrelia can “exert significant forces” to return to the bloodstream. In the tick, the bacteria has to travel in similar ways, making its way from the midgut to the hemolymph before positioning itself into the salivary glands.
Amazingly, Belperron explained, Borrelia knows where in the tick it is located and also knows whether it is moving into or out of the arthropod. This motility helps it avoid the immune system and survive in animals’ blood and tissues.
Sukanya Narasimhan, a researcher in the lab of Erol Fikrig in the Yale Department of Internal Medicine, examines vector-host interactions in the context of infectious disease. She helped illuminate why Borrelia prefers tissues to blood in both ticks and humans. In some ways, it is a “blood-borne pathogen that doesn’t particularly like blood,” she said. In the short term, blood exposure is tolerable for Borrelia while long-term existence in the blood exposes the spirochetes to “deleterious blood-meal components.”
Changing A Tick’s Gut Bacteria
Narasimhan and colleagues from the School of Epidemiology and Public Health and the Center for Medical Informatics at the Yale School of Medicine posed the following question: How does altering the composition of the tick’s existing gut microbiome, which is comprised of the commensal bacteria, affect the colonization ability of the disease-causing pathogen, Borrelia? To find out, the researchers induced a state of dysbiosis in the ticks: By rearing and maintaining tick larvae in sterile conditions, they altered the composition of the microbial community living in the arthropod’s gut.
They found that Borrelia colonizes dysbiosed larvae less than it colonizes larvae with unaltered microbe communities. In addition, their results showed that dysbiosed larvae had lower levels of the signaling protein STAT, involved in arthropod gut immunity. Interestingly, decreasing STAT expression also decreased Borrelia colonization. The observation was initially puzzling: The immune system works to shield an organism from invading pathogens, so why would a less active immune system from lower STAT levels result in less colonization by Borrelia?
According to Narasimhan, researchers had similar questions until they figured out, in this case, that STAT has a more relevant role: tissue repair and remodeling. This became evident when decreased STAT expression compromised the epithelium renewal by thinning a specific membrane that lined the tick’s gut. Similarly, decreased STAT expression resulted in decreased expression levels of peritrophin-1, a protein important for maintaining the integrity of this membrane. The fact that Borrelia, though blood-borne, prefers to reside in tissue explains this phenomenon. Narasimhan and colleagues wrote that if the membrane were compromised, Borrelia “could risk exposure to deleterious blood-meal components.”
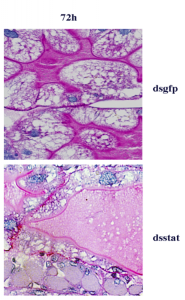
These findings demonstrated how Borrelia interacts with the tick’s microbiome, but there is much left to be explored. Instead of just perturbing the composition of commensals in dysbiosis, researchers can try to increase the percentage of a specific bacterial genus, like Rickettsia, to see how this change affects the colonization of Borrelia. The bacteria could compete with each other for nutrients, cooperate, or exchange DNA using conjugation or other forms of horizontal gene transfer. As the paper remarks, “such an understanding might catalyze ways to control vector-borne pathogens.”
The Challenges With Ticks
Despite this optimism, Giovanna Carpi, a postdoctoral associate and resident expert on tick microbiota in a vector ecology laboratory at the Yale School of Public Health, maintained that the field still faces unique challenges. One significant challenge of studying tick microbiota, she explained, is “the preservation and isolation of high quality genomic DNA and RNA from a tiny arthropod or from its organs.” Similarly, shallow sampling or sequencing methods might detect an insufficient abundance of bacterial taxa.
Belperron also teaches a class at Yale College called “Malaria, Lyme, and Other Vector-Borne Disease.” She noted that a holistic explanation of Lyme disease can be challenging because “you need a complete understanding of the immune system, microbiology, entomology, and ticks.”
“It’s impossible to be an expert on all those things in… a semester,” Belperron said.

Those who study Lyme disease face other, more philosophical challenges. In an age where global health studies are very prominent, Lyme disease is often characterized as a first world illness, considering that it primarily affects a Northeastern population. “I think it would be nice to work on a disease that affects a larger population,” said Narasimhan. “But I think tick-borne diseases are also present in the third world…When I do Lyme, I’m not saying I’m only looking at or understanding Lyme; it’s a model. Some of the lessons that you learn from Lyme disease are transferable to other disease like malaria.”
In fact, the initial inspiration for this project was the vast amount of insight required to study the human and mammalian microbiome. There remains much to be explored on how a vector encounters a pathogen and becomes a vehicle for spreading disease. Researchers hope that continuing to study the metazoan gut will provide more answers to the tick enigma.
