Tuberculosis. Cholera. Meningitis. Historically, scientists have been racing against the clock to find cures for such epidemics. What if such bacterial infections could be treated using one universal technique in only a few hours?
Such a method of treatment may become possible due to the work of a team of biomedical researchers at Harvard’s Wyss Institute led by Dr. Donald Ingber. His team has invented an external device that works like the human spleen in that it is able to filter human blood for fungi and bacteria. The device contains two compartments. The blood flows into the first chamber, which mixes the incoming blood with protein-coated nanoparticles. After the nanoparticles have bound the pathogens, the blood flows into the second chamber, which uses magnets to separate the pathogens from the blood. The purified blood then exits the device and returns to the body.

When this artificial spleen was tested experimentally, it performed exceptionally well. In the Wyss Institute experiments, rats were injected with an array of four pathogens including as E. coli, either treated or not using the filtering device, and then observed after five hours. Eighty-nine percent of the treated group survived after the five-hour period, compared to only fourteen percent of the non-treated group. This same astonishing result was replicated when the researchers repeated this procedure in vitro with human blood. After only a few hours, 90 percent purification was achieved.
What makes this device so groundbreaking and effective is its use of protein-coated nanoparticles specific for markers found on the surface of certain pathogens. Traditional methods of treatment for bacterial and pathogenic diseases often require antibiotic bombardment. This technique, though effective, can cause both the rise of antibiotic-resistant bacteria and an immune response called sepsis, in which the toxins released by the dead pathogens can trigger a possibly fatal inflammatory response.
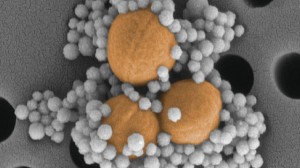
Using nanoparticles as a delivery method, the new device is able to sidestep these issues. These nanoparticles are coated with a protein called mannose-binding lectin (MBL) that is found naturally in the immune system. MBL can bind the surface glucose markers present on many different types of harmful pathogens as well as on the toxins produced by dead pathogens, which helps prevents the possibility of sepsis. The artificial spleen is also one of the first external blood purification devices to target pathogens without causing blood coagulation or a decrease in platelet count.
Though it is clear that there are many possible medical applications for such a technology, the artificial spleen still has a few key limitations and complications that must be further investigated for the technique to become commonplace. Though MBL binds to a multitude of different bacteria and fungi, there are still multiple strains of bacteria that do not have mannose and therefore cannot be bound. The use of the artificial spleen could then artificially select for such non-MBL binding bacteria and result in bacterial resistance, further complicating infection and illness.
In order to combat and further study these issues, Ingber’s team will begin to conduct trials on pigs, and human trials are anticipated in the next few years. If the technique can be extended effectively to viruses, epidemics like Ebola could possibly be treated within a matter of hours, which would revolutionize global health as we know it.
