In 2001, Cerivastatin, a drug used to lower cholesterol, was abruptly withdrawn from the market. The drug was not found to cause any problems during its preclinical trials on mice, but when given to humans, it caused 52 fatal cases of a condition called rhabdomyolysis, the rapid breakdown of skeletal muscle tissue. Animal testing had failed to effectively screen for potential complications of the treatment, which is not an uncommon problem in biomedical research.
If preclinical trials could be performed on human tissues outside of the human body, treatments for a wide range of medical disorders could be made much more effective and safe. With the development of the first in vitro human skeletal muscle that responds to electrical and chemical stimuli, researchers at Duke University are already making progress in this direction. The study, led by associate professor of bioengineering Nenad Bursac and post-doctoral student Lauran Madden, holds promise for improving the efficacy of preclinical trials in determining which drugs are safe and which are potentially hazardous. This new development could also enable more personalized treatments for a variety of conditions.
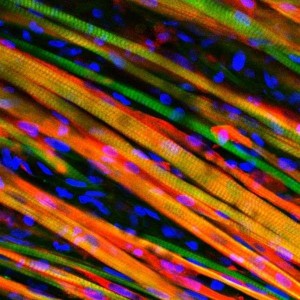
In the human body, muscle tissue formation begins when individual cells called myoblasts fuse together to form long muscle fibers called myotubes. Then, these myotubes, which generally run parallel to the muscle’s longitudinal axis, align into specific structural patterns to compose muscle tissue. Prior to Bursac’s study, researchers had managed to expand human myoblasts and form test-tube myotubes. However, these two-dimensional models could not mimic human muscle, since the fibers responded to stimuli individually rather than as a cohesive, three-dimensional unit.
To construct 3D in vitro tissue, Bursac and Madden attempted to replicate the way that muscle tissue is grown within the human body. First, they obtained and regenerated samples of myoblasts from human biopsies and surgical waste. Then, they poured a solution containing these cells, as well as a nourishing gel solution, into a scaffold structure of molds attached to a frame. This set-up allowed the individual myoblasts to align and form myotubes. Over the next two weeks, the myotubes began to arrange themselves into a three dimensional structure that constituted real muscle tissue completely outside the human body.
To evaluate how closely this in vitro muscle resembled actual human muscle, the researchers performed a battery of tests. First, they determined that the in vitro muscle, like human muscle, could contract effectively in response to electrical and chemical stimuli. Next, they studied how the muscle model handled calcium. They found that in response to chemical and electrical stimulation, calcium passed through cell membranes into the in vitro muscle cells just as it does in human muscle cells.
Lastly, the scientists compared the in vitro and human muscles’ responses to three different classes of drugs. Like human muscle, in vitro muscle contracted with less force in response to two types of statins, drugs commonly prescribed to prevent coronary artery disease. Both muscles also exhibited weaker contractile force after exposure to chloroquine, an anti-malarial drug, and β2 adrenergic agonists, which are commonly prescribed to patients who suffer from asthma. These tests further demonstrated the ability of the laboratory tissue to model real human muscle.
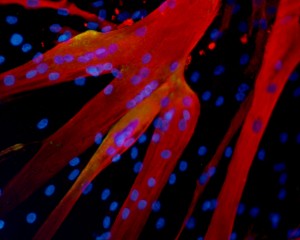
Bursac and Madden’s study has important implications for the future of drug testing. Since their in vitro muscle tissue closely resembles human muscle, it could more accurately represent treatment outcomes in preclinical trials than animal testing currently does. The in vitro muscle could also lead to more individualized treatment programs. For example, experiments performed on in vitro tissue grown from a patient’s biopsy could help determine which drug combinations best address the individual’s specific medical needs.
Currently, Bursac is working with professors at the Duke School of Medicine to compare the effects that drugs have on patients with the effects that they have on his in vitro muscle models. With hope, the use of lab-grown human tissue to test novel drugs and therapies will become a common and life-saving practice in biomedical research.
Cover Image: This is a microscopic image of engineered heart muscle cells that have been stained. Image courtesy of Nenad Bursac, Duke University.
