Approximately every minute, someone in the United States develops Alzheimer’s disease. With over 5 million Americans currently diagnosed, Alzheimer’s is the sixth leading cause of death in the United States and is associated with declines in memory and cognitive function. The disease takes an immense physical, emotional, and monetary toll on patients and their caregivers, with hardship only increasing as the illness progresses.
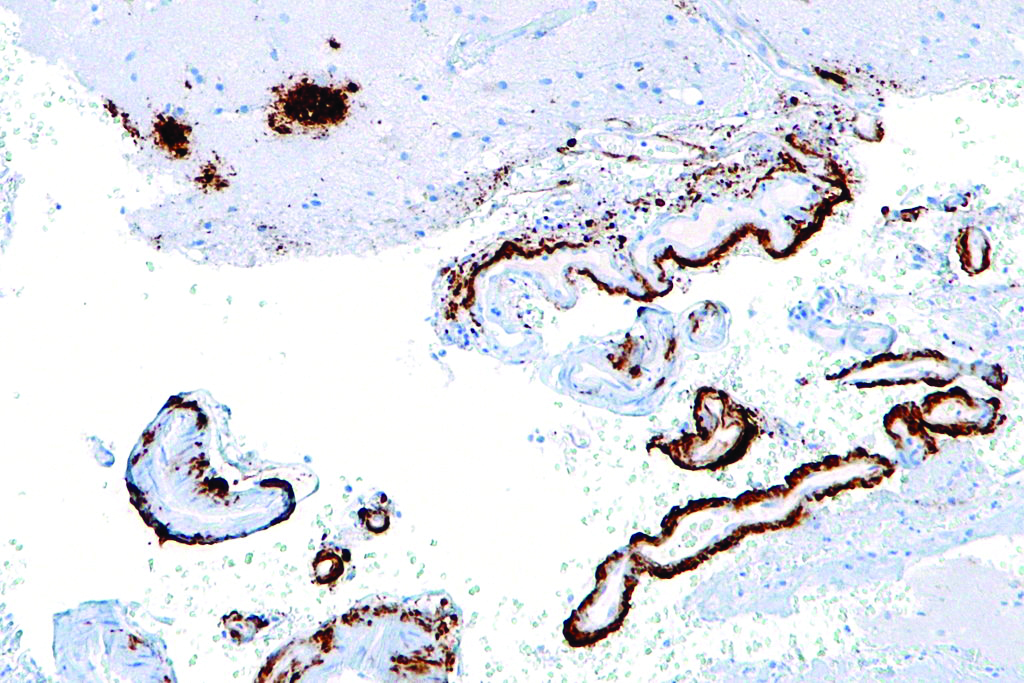
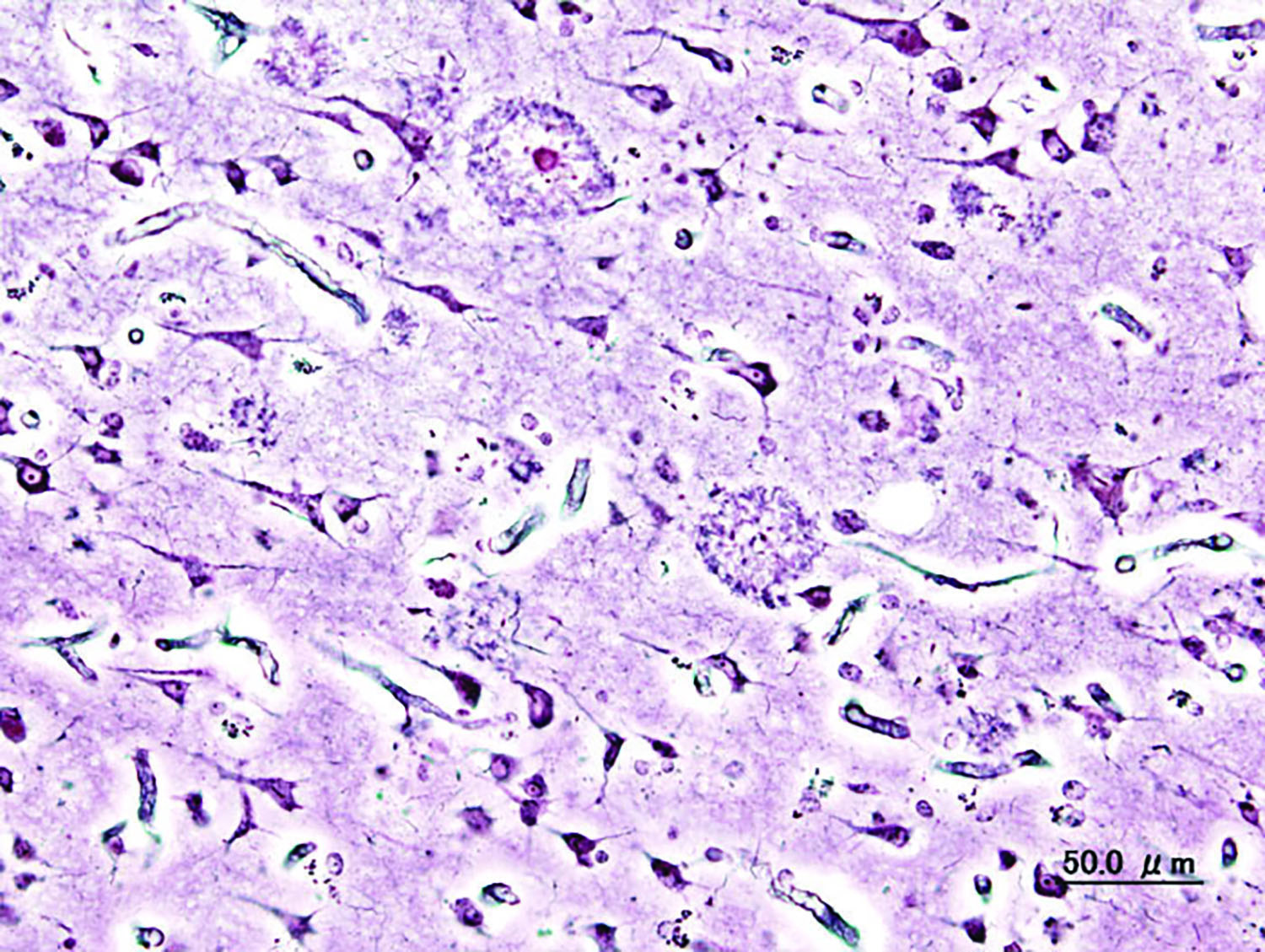
There is no cure for Alzheimer’s disease, and until recently the outlook for treatment was grim. In a recent study led by researchers at Biogen, a biotechnology company focused on treating neurological diseases, scientists used a human antibody to target and destroy amyloid-β, a large molecule associated with the neurological symptoms of Alzheimer’s. Within the brains of Alzheimer’s patients, amyloid-β molecules aggregate in two different forms: large, insoluble plaques and smaller, soluble complexes. Of these, the latter have been demonstrated to be neurotoxic and causative of cognitive dysfunction. These soluble complexes bind to the junctions between neurons, called synapses, and interfere with their ability to communicate with other cells. Furthermore, binding by amyloid-β may elicit an adverse immune response, causing the body to destroy its own non-communicating neurons.
Previous attempts at harnessing the disease-fighting ability of the immune system to treat Alzheimer’s have been ineffective. Aducanumab, the antibody developed by researchers, binds effectively to both soluble and insoluble aggregates of amyloid-β and appears to successfully reduce plaque size in human clinical trials. These amyloid-β plaques are not implicated as the primary cause of Alzheimer’s symptoms, but their size corresponds to the amount amyloid-β in a patient’s brain. Since plaque size is more easily measured than the concentration of soluble amyloid-β complexes, researchers use plaques to monitor disease progression. In fact, the aducanumab-induced decreases in plaque size observed in this study were accompanied by astounding changes in disease progression.
After initial biochemical experiments demonstrated that the antibody could bind to amyloid-β, researchers tested aducanumab in mice. The mouse trials confirmed that the antibody was able to enter the brain and clear amyloid-β aggregates. After obtaining these encouraging results, researchers began human clinical trials. The recently completed phase-one trials included a relatively small number of participants, but appeared to show noteworthy results. After receiving aducanumab for one year, patients showed significant reductions in amyloid-β plaque size and maintained their cognitive abilities and memory better than those given a placebo.
Since the trial’s conclusion of in 2015, Biogen’s aducanumab research has increased in scale: researchers are currently testing the therapy in two large clinical trials. They aim to gather more information about the antibody, including its ideal dosage and potential side effects. Christopher Van Dyck, Director of Yale’s Alzheimer’s disease Research Center and the principal investigator for aducanumab trials at Yale, believes that aducanumab will likely enter the medical market if the same efficacy of treatment can replicated in these larger trials. “I think that these results were unprecedented in many ways in our field, and I think that they are game-changing,” explained Van Dyck.
Although the initial clinical trial was a small, early-phase study, it accomplished something groundbreaking. It provided solid evidence for the effectiveness of immunotherapy against Alzheimer’s disease. Future medical and pharmacological research will undoubtedly incorporate this valuable insight in the battle against Alzheimer’s.