A receding hairline, a wrinkly forehead, and a saggy butt are considered undesirable hallmarks of aging, according to representations in popular media. When people think about anti-aging, they usually think about reversing these surface-level effects. Everyone has seen beauty campaigns promising to tighten one’s skin or prevent balding, but the larger causes and effects of aging go far beyond the symptoms in one’s appearance.
While it cannot be argued that the dramatic increase in the human lifespan over the past five hundred years reflects positive advancements in medicine, hygiene, and other health systems, a longer life comes with an increased risk of chronic disease. Age is the biggest risk factor for every chronic disease we know of, including cancer, cardiovascular disease, and neurodegeneration, but three different specialists treat each one. People are not getting older in a healthy way in large part because modern medicine does not treat aging in a disease in and of itself.
Professor Vishwa Deep Dixit and post-doctoral fellow Christina Camell of the Yale School of Medicine took up this challenge and worked to understand aging and its effects together as one connected disease. They focused on inflammation, the body’s response to wound or injury, which is known to be a primary trigger of age-related disease. While in young people, an immune response will respond to infection and then subside, in the elderly these patterns of inflammation never abate, which causes stress and dysfunction. Dixit and Camell drew a link between this inflammation and the fat accumulation also characteristic of aging. The nexus of this connection lies in macrophages, which are specialized immune cells. The researchers found that a class of hormones called catecholamines directly communicates with a previously undiscovered subset of macrophages called nerve-
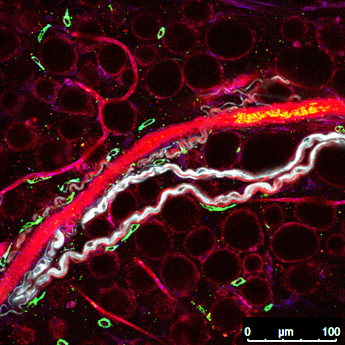
associated macrophages, which use the nerves to promote lipolysis, or fat breakdown. Sustained inflammation in aging increases concentrations of proteins that break down these catecholamines, which, in turn, prevents lipolysis. In other words, as the mice they used aged, an inability to clear inflammation decreased catecholamine levels, which resulted in fat accumulation. This research implicates crosstalk between the immune, nervous, and metabolic systems in the disease of aging, which may provide new avenues for research to promote wellness in the elderly.
The deficit
To understand the immune system’s role in aging and lipolysis, Camell and Dixit initially needed to flesh out the intersection between these two processes and the role catecholamines played in them. Two-year-old mice, the equivalent of 65-year-old humans, release less fat from their adipose tissue, or fat reserves, than did young ones. Surprisingly, when the aged mice were given catecholamines, their rate of lipolysis and consequent fat release returned to levels seen in young mice. When cells that store fat called adipocytes were taken from these aged mice and grown alongside macrophages, however, the bounce-back in lipolysis was no longer observed. Conversely, the addition of young macrophages to old fat restored lipolysis. These results were a first clue: catecholamines promote lipolysis, but something about aged macrophages specifically reduces this breakdown of fat. The first major finding, therefore, was that macrophages in the adipose tissue control the decrease in lipolysis observed in aging.
The next step was to figure out the specific mechanism by which this deficit in fat breakdown occurs. The researchers analyzed gene expression in aged macrophages and found that they express more genes implicated in catecholamine metabolism than do young ones, which solidified the link between the age of the macrophages and their ability to facilitate lipolysis with catecholamines. One protein associated with both aging and inflammation is NLRP3, which forms part of a larger complex called the inflammasome. Dixit and Camell found that the activation of the NLRP3 inflammasome prevented the release of fats from adipose tissue. On the other hand, when the researchers engineered mice without the gene responsible for the production of NLRP3, they were able to break down fat normally even as they aged.
Chit-chat
The researchers wanted to see if they could uncover an underlying biological mechanism that would explain these observations. To accomplish this goal, they performed RNA sequencing analysis, which gives a complete picture of gene expression, and saw that the set of genes responsible for catecholamine degradation in aging macrophages was dependent on NLRP3. Sustained NLRP3 inflammasome activation—sustained inflammation—thus, seemed likely to shift the gene expression profile of macrophages towards the aged one. A gene called GDF3 that controls fat accumulation and showed the strongest positive correlation with aging was reduced down to the level of young mice when NLRP3 was knocked out, which corroborates this evidence. The NLRP3 knockout also showed restored-to-normal levels of genes implicated in fat breakdown and catecholamines’ proper functioning. On the flip side, this result indicated that an increase in NLRP3 and its resultant inflammation in aged macrophages promoted the degradation of catecholamines that results in reduced lipolysis.
To visualize the location of macrophages relative to catecholamines, the research team used reporter mice, which are mice specially engineered to have fluorescent proteins in specific cell types. Using these markers, Camell was surprised to find macrophages in a pattern never previously observed. “The macrophages are actually lining the nerve,” she said. “They almost look like they’re hugging it.” This configuration led Camell to conclude that she had discovered an entirely new subset of macrophages, termed nerve-associated macrophages, that represents the intersection of the nervous, immune, and metabolic systems. These immune cells directly access the catecholamines from the nerves, allowing them to regulate levels of these hormones in the adipose tissue. Whereas before the exact relationship between the nerve, the released catecholamine, and the adipocyte was unclear, now macrophages have been established as a direct and crucial link. This immediate connection allows the nerve-associated macrophages to influence both lipid metabolism and inflammation, two primary factors that contribute to aging, via their control over the concentration of catecholamines present in the adipose tissue.
Learning how to manipulate this system was a final step to cement the validity of these findings. Aged macrophages were found to overexpress an enzyme called MAOA that is implicated in catecholamine degradation, so the researchers treated aged mice with a MAOA inhibitor and saw increased fat breakdown. This result confirms the link between catecholamine degradation and resistance to lipolysis in aging. To sum up the model, when catecholamines are released by still functional nerves in aged individuals, inflamed macrophages have excess biological “machinery” like MAOA that degrades the catecholamines, so the hormones are broken down before they can act on the adipocyte to promote lipolysis. This more complete picture gives researchers a better comprehension of the complicated crosstalk between the nervous, immune, and metabolic systems and how it influences fat breakdown in aging.
The life in your years
Down the road, an understanding of these intersecting communication patterns has far-reaching applications in treatment for aging in line with its classification as a chronic disease. The aforementioned class of MAOA inhibitors is currently used as treatment for depression, but if research worked to localize these drugs such that they do not affect the brain, this therapeutic avenue would be promising. With lower levels of MAOA comes higher levels of catecholamines, which can restore lipolysis in aging individuals. An alternative target is GDF3, the NLRP3-dependent protein that inhibits fat breakdown. While GDF3 is a single upregulated protein under the overarching control of the NLRP3 inflammasome, NLRP3 itself is required for fighting off infection, so drugs to block this protein can increase the risk of certain infections. Instead, neutralization of GDF3 is an indirect way to achieve the same goal. If aberrant inflammation via GDF3 can be prevented, cells of the adipose tissue will show better lipolysis. Regardless of the mechanism, these strategies work to influence the crosstalk between macrophages and their associated nerves, which, in turn, influences fat metabolism. The communication between the nervous, immune, and metabolic systems that this research establishes integrates almost every organ system and so has clear potential to address the disease of aging as a condition that impacts all aspects of human health.
When viewed through an even broader lens, this research is about improving people’s healthspan, the time during which an individual is in good health. To clarify the relationship between catecholamine degradation and inflammation is to draw the links between the immune, nervous, and metabolic systems that, for example, can be implemented to mitigate neurodegenerative and other age-related diseases. Ultimately this research may represent a key to healthier aging. “Our goal is not to simply increase lifespan,” said Dixit, “but to add life to the years that exist.” An understanding of the mechanisms controlling lipolysis, however narrow of a focus, can help to achieve this universal ideal: to add life to your years.
About the Author
Charlie Musoff is a sophomore in Davenport College and a molecular, cellular, and developmental biology major. Besides being Yale Scientific’s Outreach Designer, Charlie enjoys running, singing with the Baker’s Dozen, and teaching with Community Health Educators.
Acknowledgements
The author would like to thank Christina Camell and Professor Vishwa Deep Dixit for their time and insights.
Further Reading
Pirzgalska, Roksana M, et al. “Sympathetic Neuron-Associated Macrophages Contribute to Obesity by Importing and Metabolizing Norepinephrine.” Nature Medicine, 9 Oct. 2017, doi:10.1038/nm.4422.

