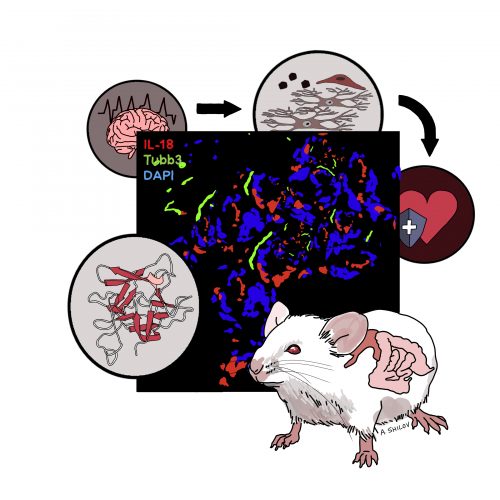
Illustration by Anasthasia Shilov.
When we think about infection, we think about microscopic drama: the immune system as the sole guardians of our bodies; white blood cells as staunch defenders against pathogenic invaders. However, while B cells hunt for antigen-presenting cells and phagocytes swallow bacteria whole, there has been a hidden accomplice in our bodies’ defense from infection: the nerves that line our intestines.
In contrast to the bacteria-destroying immune system, the intestines harbor the peaceful presence of commensal microflora, friendly gut bacteria that help the body digest food and produce vitamins. However, the amicable existence of the gut microbiota survives in delicate balance with the ruthless elimination of pathogenic microbes. This complex equilibrium relies on signaling between cells in the immune system and intestinal cells that form the mucosal barrier, which segregates gut bacteria from immune cells to avoid unnecessary immune responses. Until now, scientists have thought that these two cell types were the lone ringleaders in the synthesis of bactericidal proteins and molecules that ward off threatening infections.
Earlier this month, scientists at the Yale School of Medicine, in collaboration with researchers at Harvard Medical School, discovered that neurons in the intestinal nervous system play a crucial role in governing immune response in the intestines. Moreover, they observed that this antimicrobial function of intestinal neurons was non-redundant, potentially revealing another key player in the struggle against infections of the intestine.
What is the Enteric Nervous System?
There are a variety of different cell types in the intestine. Epithelial cells form the border on the inside of your intestine where food passes through, while outside intestinal tissue contains immune cells that detect and target bacteria. Until now, the wide network of intestinal neurons, called the enteric nervous system (ENS), was often overlooked in gut immunity research.
“There are actually more neurons in your intestine than in your brain, so it’s very neuron-dense,” said Abigail Jarret, first author of the paper and a PhD candidate in Immunobiology at the Yale University School of Medicine. The ENS has even been described as a “second brain,” able to operate autonomously and communicate with the central nervous system to maintain the delicate homeostasis in the intestine.
The brain-gut axis, referring to the bidirectional link between the central nervous system and the ENS, has recently become a point of conversation in the scientific community. Because of this, scientists have also begun to explore how the nervous system might influence the immune response to infection.
“Up until maybe five years ago…we [made] these beautiful plots of the intestines, and we only put epithelial cells there as a reference for the structure, and … everything else was immune cells and empty space,” said Esen Sefik, a postdoctoral fellow at the Yale School of Medicine and a co-author of the paper. But the community started to become interested in that empty space. Over time, scientific interest in the intestine grew from epithelial cells to other cell types, and more recently, cells in the nervous system. This study stemmed from an interest in understanding the relationship between epithelial cells—the first line of defense against infection by bacteria in the gut microbiota—and neurons in the ENS.
Single Cytokine, Substantial Significance
Hirschsprung disease is a birth defect characterized by missing nerves in the intestine. A characteristic complication of this disease is inflammative overgrowth of the microbiota, which indicates a profound relationship between the ENS and the mucosal barrier. Taken with studies reporting that the ENS contributes to intestinal inflammation, responds to pathogenic infection, and triggers effects on bactericidal protein secretions by epithelial cells, the authors of the study were inspired to investigate the role of the ENS in regulating mucosal barrier immunity in the intestine.
In immune cells, cytokines act as chemical messengers that cells use to talk to each other, in order to regulate the immune response. An especially important cytokine in the separation of the gut microbiota from the immune system is Interleukin-18 (IL-18), which helps to recruit other cells to clear bacterial infection. Around thirty years ago, scientists genetically modified the first mouse that completely lacked the ability to produce IL-18. When they infected it with intestinal salmonella, it died. Many years later, a version of the mouse was created that lacked IL-18 only in specific cells, allowing researchers to further study the role of different cell types in the immune response.
“I think people become very interested in the function of a single cytokine or a single molecule because as biologists, we want to understand why we evolved to have it,” Jarret said. “Why is lacking one thing making these mice so sick? When we had the ability to delete this gene in specific cell populations, we could start to understand the contribution of that molecule from different cell sources.”
Secretion and Deletion
To further understand how different sources of IL-18 contribute to protection against infection, the researchers tested mouse strains that lacked IL-18 in different cell populations. They infected each strain with Salmonella typhimurium, a bacterium that targets the intestine, typically acquired through contaminated water or food. When mice could not produce IL-18 in their epithelial and immune cells, they were not susceptible to salmonella infection. Surprisingly, when IL-18 was deleted from intestinal neurons alone, mice displayed symptoms associated with salmonella infection. This helped the researchers determine that neuronal IL-18 is essential for preventing bacterial infection in a way that epithelial and immune cells cannot.
Next, the researchers sought to determine how IL-18 protected mice from bacterial infection. The researchers used RNA sequencing (RNA-Seq) to understand whether unique cellular signaling events were present in neuronal IL-18-deficient mice compared to mice that lacked the cytokine in other cell types. Using RNA-Seq, the researchers could determine the presence and quantity of RNA in intestinal tissue samples to analyze differences in gene expression between different IL-18-deficient mice strains. RNA-Seq revealed that in mice lacking IL-18 secretion from neurons, the expression of bactericidal and antimicrobial genes that produce proteins vital to the immune response were exclusively reduced, rendering the mice susceptible to salmonella infection. These proteins are called antimicrobial proteins (AMPs), which constitute a major arm of the innate immune system. AMPs form holes in bacterial membrane, which kills bacteria.
“So, IL-18 produced by neurons in the intestine was instructing epithelial cells to make these antimicrobial proteins,” Jarret said, “and if you got rid of this IL-18, this communication was no longer occurring, and the mice didn’t have efficient production of antimicrobial proteins and became susceptible to infection.” Through their experimentation with IL-18, the researchers had described a novel pathway of communication between neurons in the intestine and epithelial cells.
Coexisting with Hostile Neighbors
The unearthing of this obscure communicative route calls for a plethora of further research. “Molecularly, we don’t know how the IL-18 is being produced…we’re really interested in what’s inducing the IL-18 from neurons, and we think it’s going to be something produced by bacteria in our microbiota,” Jarret said. Currently, there is scientific interest in what molecules the microbiota produces and how they can act as signals to other cells in the intestine. She hopes that the study will help people remember that considering these non-classical immune cell subtypes is still important in understanding the immune response. “I think that’s really in vogue right now,” Jarret said.
The findings of the study also provide novel insights into diseases of the intestine. For example, a number of disorders can induce neuron death in the ENS, causing overgrowth of bacteria or an imbalance in the number or kinds of bacteria in the gut, leading to impairment of the microbiota. This study uncovers connections between neuronal IL-18 secretion and the mucosal barrier’s meticulously cultivated separation between the immune system and gut microbiota.
“It could be really important in maintaining that boundary and allowing us to coexist with them,” Jarret said. Intestinal diseases such as irritable bowel syndrome and small intestinal bacteria overgrowth can cause inflammation due to gut bacteria migrating too close to epithelial cells. This induces common symptoms such as diarrhea, weight loss, and abdominal pain. “It’s possible that in those circumstances, if we could figure out a way to induce IL-18 production in neurons that maybe [we could increase] antimicrobial protein production to help create space between ourselves and bacteria,” Jarret said.
The study may also open new doors to tackle antibiotic-resistant strains of salmonella and other food-borne infections. The rapid emergence of resistant bacteria has been called one of the biggest threats to global health by the WHO. Evolution of antibiotic resistance in foodborne pathogens makes infections like salmonella and E. coli impossible to treat, adding to the 400,000 deaths worldwide caused by foodborne disease each year. “[The study] might open up new avenues of controlling these infections rather than looking for an antibiotic that they can get resistant to,” Sefik said.
Above all, the study reminds us of the value in thinking more broadly about the immune system and considering how different systems are interacting within the body. The unexpected findings of the study demonstrate a relationship in human physiology scientists have rarely thought about before. Sefik emphasizes the importance of partnerships between different specializations in biological research. “I love the collaborative aspect of this [study]. I think that neuroscientists and immunologists should start collaborating more. It’s happening, but really only at places like Yale,” Sefik said. Through such interdisciplinary work, the scientific community may finally be able to elucidate modes of biological communication, like those between the ENS and the immune response, that could enable the treatment of complex human disease.
About the Author
Christina Hijiya is a sophomore MCDB and HSHM major in Davenport College. In addition to writing for YSM, she is a member of WORD: Performance Poetry at Yale, volunteers at HAVEN Free Clinic, and teaches for Community Health Educators.
Acknowledgements
The author would like to thank Abigail Jarret and Esen Sefim for their time and thoughtful comments about their research.
Extra Reading
Flayer, C. H. & Sokol, C. L. (2020). Nerves of Steel: How the Gut Nervous System Promotes a Strong Barrier. Cell 180(1): 15-17.