It may seem like a simple concept—bacteria have to be able to move in order to infect their host. However, for decades, scientists have been scratching their heads, attempting to uncover the intricate workings of the molecular motor mechanisms that allow bacteria to move in their signature “run-and-tumble” pattern. The complex that allows bacteria to achieve this movement, known as the bacterial flagellar motor, is of particular importance due to the important effect it has on the ability of potentially life-threatening bacteria to infect organisms. In a recently published in Nature Structural & Molecular Biology, researchers proposed a novel mechanism by which they believe the spirochete Borrelia burgdorferi, the causative agent of Lyme Disease, switches the rotation of its flagellar motor from counterclockwise (which allows bacteria to move in long runs), to clockwise (which allows bacteria to randomly tumble).
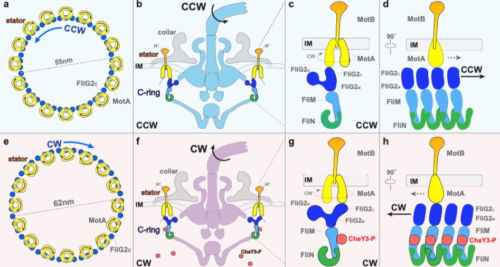
In this study, researchers were able to visualize high-resolution flagellar motor structures in two mutant strains of B. burgdorferi. The strains of bacteria, ∆cheX and ∆cheY3, were mutant for two types of chemotaxis proteins, which regulate the direction of flagellar rotation. In each of these mutants, the bacteria were locked in counterclockwise or clockwise rotations, respectively. In order to resolve the structures of the bacterial flagellar motor, as well as visualize the changes that occurred when the chemotaxis proteins were subjected to varying conditions, the researchers used a combination of cryo-ET imaging, subtomogram averaging, and genetic modification of the bacterial cells.
After visualizing the flagella motor structures, the researchers noticed that their results contradicted previous findings. “Before we resolved the high-resolution flagellar motor structures, we had some expectations on the structures based on the previous publications,” said Yunjie Chang, a researcher at Yale University who authored the paper. Chang pointed out a few major surprises. First, the team noticed that the flagellar rotor ring subunits form a “hand-in-hand” structure at the base, as opposed to a “donut structure” that had been proposed in previous work. In addition, Chang noted that the stator unit, the part of the flagella that powers the motor, remains the same regardless of the direction of rotation. This contradicted previous research, which had suggested that the conformation of the stator unit changed along with the direction of rotation of the flagella. In the end, these novel pieces of structural information led researchers to propose a novel mechanism. “I think [that] is really the intriguing part of doing structural biology research,” Chang said.
The mechanism proposed by the authors is as follows: the phosphorylation of cheY3 to cheY3-P proteins promotes binding to FliM, a switch protein that forms the rotor of the bacterial flagella. This complex then interacts with two other chemotaxis proteins, cheY and cheZ; these domains interact with other components of the motor that are able to determine the direction of flagellar rotation as either clockwise or counter-clockwise. This contact then induces the remodeling of another switch protein, FliG2. The conformational changes in FliG2 result in an inward flow of H+ ions causing the torque generator of the bacterial flagella to rotate. The interaction between the torque generator and the switch complex allows the flagella to switch rotational direction. While scientists previously had observed that the binding of the cheY protein to the FliM switch protein induced the shift from counterclockwise to clockwise rotation, the exact mechanism behind the rotational switching was unknown.
While this is indeed a fascinating mechanism, the researchers acknowledge that much more work is needed in order to confirm or deny this theory of rotational switching in bacterial flagella. If proven true, this theory ultimately helps us to understand the mechanism behind one of the most interesting and efficient pieces of molecular machinery. “The flagellar motor itself is a complex and fascinating nano machine, which can convert the electrochemical potential difference across the cell membrane into mechanical work with almost [full] efficiency, and can rotate as fast as 1,700 revolutions per second, which is much faster than that of a Formula One racing car engine,” Chang said. By better understanding this intriguing mechanism, scientists may be able to better understand bacterial virulence and invasion of host organisms.