The molecular instructions involved in building an embryo are complex and beautiful, but not fully understood. Through studying zebrafish embryogenesis in the Nicoli Lab at Yale, postdoctoral fellow Dionna Kasper recently discovered a new pathway that regulates the early development of the cardiovascular system in embryos. This provides exciting possibilities for improving therapies, such as bone marrow transplants for blood disorders.
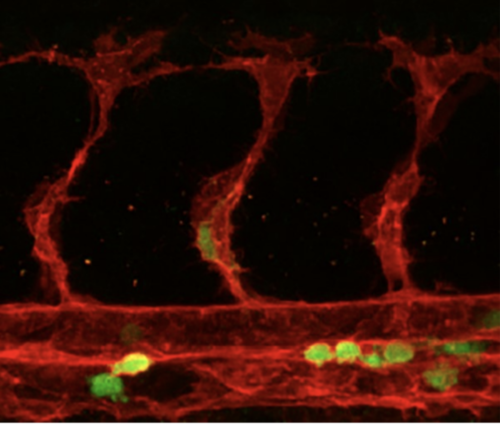
In vertebrate embryos, the cardiovascular system first begins to form when some endothelial cells, which are the internal lining of blood vessels, turn into precursor cells responsible for blood production. Those special endothelial cells are known as hemogenic endothelial cells (hemECs), and the precursor cells are called hematopoietic stem and progenitor cells (HSPCs). The process by which the hemECs turn into HSPCs is known as the endothelial-to-hematopoietic transition (EHT). After the EHT, the HSPCs delaminate from the lining of the blood vessels and enter circulation to give rise to all the blood cell types in the body.
The factors that regulate the EHT process are not completely understood. However, Kasper uncovered the previously unknown role of miR-223, a molecule that belongs to a family of small, noncoding RNAs that silence gene expression. Kasper explained that this study was focused on embryonic development because that is the only window of time when HSPCs are produced from scratch. When she deleted the gene coding for miR-223 in mutant zebrafish and mice embryos and observed elevated levels of hemECs and HSPCs, Kasper concluded that miR-223 inhibits the production of those types of cells.
Specifically, miR-233 affects the EHT by repressing expression levels of N-glycoenzymes alg2 and st3gal2. N-glycoenzymes are proteins involved in N-glycosylation, a process by which sugars are added to proteins. These sugar chains are crucial to protein folding, function, and signaling. The sugar modifications that alg2 and st3gal2 add to proteins limit EHT and cell proliferation. Fascinatingly, there has not yet been past research associating N-glycosylation with EHT, so this exciting discovery about the role of a “sugar code” in cardiovascular cell development provides novel information about the growth of cells responsible for cardiovascular system development.
One challenge that Kasper encountered was the amount of material needed to analyze the sugars in endothelial cells. She was unsure whether she’d be able to see the sugars or find proteins modified by N-glycosylation. Ultimately, though, she was able to do both, thus finding a new pathway involving N-glycan sugars that regulate the production of HSPCs.
“These cells have incredible therapeutic potential for patients with leukemia or other blood disorders to help them regenerate their blood system, but there’s a shortage of ways to get hematopoietic stem cells,” Kasper said. With the new information about how HSPCs are generated in embryos, researchers can improve strategies for producing the stem cells needed for these therapies.