Image courtesy of Vijay Ramani.
Imagine an all-in-one battery that can both generate and store its own energy. Unitized regenerative fuel cells (URFCs) are devices that can, with the flick of a switch, convert hydrogen and oxygen into electricity and water, and vice versa. Bifunctional catalysts, which serve to speed up both reactions, are required in order for URFCs to be effective. One such bifunctional catalyst, Pt-pyrochlore, was discovered by a team led by Vijay Ramani, Roma B. and Raymond H. Wittcoff Distinguished University Professor at Washington University in St. Louis. Ramani has researched these hydrogen-oxygen fuel cells since his first year as a PhD student in Chemical Engineering at the University of Connecticut—and has been enthralled ever since.
The two reactions performed by URFCs are the fuel cell reaction and the electrolyzer reaction—exact opposites of each other. The former involves combining hydrogen and oxygen to generate water and electricity, while the latter is the reverse process, whereby water is split back into hydrogen and oxygen by adding electricity. “If you operate in one polarity, you combine hydrogen and oxygen to make water with electron flow, and if you switch the polarity, you can input electricity and split the water to give hydrogen and oxygen,” Ramani said. Thus, this singular device can easily switch between generating fuel from water and electricity and converting the fuel into electricity and water.
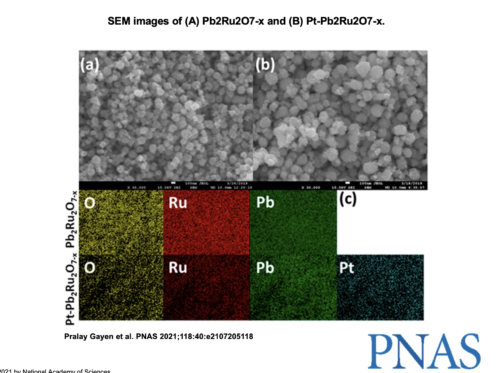
Ramani explained that the reversible conversion of hydrogen into protons and electrons is not a particularly difficult process. The main challenge involves transforming the oxygen into water and vice versa, since both reactions are sluggish. Thus, effective URFCs require a bifunctional catalyst that can make both of these reactions progress at a significantly higher speed to enable practical use. Scientists already knew the effectiveness of platinum as a catalyst for oxygen reduction and lead ruthenate pyrochlore as a catalyst for oxygen evolution, the opposite reaction. Ramani and his team effectively combined these two catalysts to yield Pt-pyrochlore, testing different combinations of metal compositions and optimizing bifunctional activity, surface area, and electron conductivity.
The implications of this successful bifunctional catalyst in URFCs are far-reaching. “It’s a wonderful device for energy generation and storage,” Ramani said.
For instance, when energy sources are intermittent—such as when electricity is generated from solar or wind energy—URFCs can help buffer this inconsistent output. “When the wind is blowing, you can essentially make and store hydrogen and oxygen through water electrolysis, and when the wind doesn’t blow, you can use that hydrogen and oxygen to generate electricity to ensure a more constant output,” Ramani said. This particular advantage is especially applicable to the space sector, where energy can be stored for lengthy periods of time, while electricity is generated whenever solar energy is accessible. Ramani, however, recognizes that these devices are still too expensive to be integrated into mass consumer applications.
Now, Ramani and his team are striving to work with industry partners to test and further de-risk the technology. “It’s one thing to demonstrate something in the lab; it’s completely different to take it to practice and make a commercial unit out of it,” Ramani said. Ultimately, Ramani’s work in improving URFCs through the Pt-pyrochlore bifunctional catalyst will make energy generation and storage more feasible, efficient, and streamlined.