Image courtesy of Luna Aguilar.
With great power comes great responsibility. In an age where the world has become increasingly reliant on technology, the threat of electronic waste or e-waste—including many discarded products containing batteries—has become increasingly dire. In 2019 alone, an estimated 48.6 million tons of e-waste was generated worldwide, a rapidly expanding issue that has only continued to grow. Because standard lithium batteries contain non-biodegradable and often toxic rare earth metals, the accumulation of e-waste releases harmful chemicals that can contaminate groundwater and the air, putting the environment and the health of billions at risk.
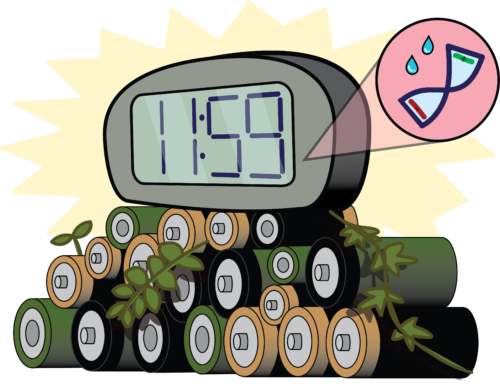
The quest for green power seeks to eliminate the threat of e-waste by harnessing sustainable materials. This pursuit fascinated Gustav Nyström, head of cellulose & wood materials at the Swiss Federal Laboratories for Materials Science and Technology. By harnessing natural, environmentally-friendly materials, researchers in Nyström’s lab were able to power the crystal display of a LED alarm clock using a disposable paper battery activated by just two drops of water.
The project, which also involved postdoctoral researcher Alexandre Poulin and doctoral researcher Xavier Aeby, sought to search for an environmentally-friendly battery high in density, important for a longer run time, that would be suitable for single-use devices such as sensors. “For instance, in biomedical industries, where many diagnostic tests are discarded after a single use due to hygienic and ethical reasons, there is a lot of plastic waste being generated,” Nyström said.
An electrochemical battery stores and discharges energy through a series of oxidation and reduction reactions that occur at the anode and cathode, respectively. In addition to these components, a separator prevents the electrodes from coming in contact and short-circuiting, while the electrolyte facilitates the movement of ions between the anode and cathode. In battery fabrication, the material choice for these components is especially important for optimized, reliable function. “We made a strict selection based on what we thought were the most promising materials in terms of energy density and stability for this development,” Nyström said. “It’s about choosing the right type of materials that are not harmful to our environment. That’s a big and important goal for us.”
The ideal material is stable and highly conductive, with low contact resistance, high energy storage capacity, and a fast charging rate. After testing many combinations, the final design used a zinc anode, a graphite carbon air cathode, a paper separator, and a water-based salt electrolyte. The electrodes were stenciled onto the paper using multi-material inks that contained a mixture of ethanol, shellac, and sodium chloride ions which are required to form the conductivity needed in the battery for electron flow. The dry batteries can then be stored indefinitely until water is added, which permeates the paper membrane, dissolving the salt ions and activating the battery within twenty seconds.
The battery inks were characterized by shear thinning behavior and yield stress, physical factors important in additive manufacturing. This method adds components by layering them and has great advantages in reducing manufacturing waste. “[Our goal is] also being responsible with the amount of material used,” Nyström said. “When you manufacture, you only need to use exactly the amount of multi-material inks that you need for those different components.”
The researchers also performed electrochemical tests on the battery. A single cell demonstrated a 1.2 V potential, which is proportional to the energy delivered to the cells. Power capability was measured to reach 150 μW, enough to power an alarm’s LCD crystal display, hearing aids, and electronic watch calculators. With more printed batteries added in series, the voltage increases, accommodating devices with greater power usage. After one hour of operation, the voltage decreased as the paper substrate dried. However, upon the readdition of water, the performance was recovered, allowing for extended battery life by increasing how much water the paper could hold.
In the future, the researchers hope to study the length of battery life following rehydration of the battery and explore further implementations of organic materials. Nyström believes that it has the potential to play a valuable part in the reduction of e-waste in powering single-use diagnostic tests and sensors, but there is still much work to be done. “We have had a lot of academic developments, and now is really the time to see what and how much can be transferred into real products,” Nyström said. “I think [paper] is quite a promising material, but we need to see what is feasible and where the best applications will be.”