Leigh syndrome is an incurable neurological disease caused by mutations to mitochondrial DNA, the circular DNA that governs mitochondria’s ability to power the cell. The disorder disrupts cellular respiration and results in rapid loss of muscle movement and mental capabilities, often leading to premature death. Leigh syndrome is passed on from mother to child by the diseased mitochondria present in the egg, making it nearly impossible for affected mothers to have healthy children.
However, there may be hope for women suffering from Leigh syndrome and other mitochondrial diseases. This hope comes in the form of the three-parent embryo, a recent scientific innovation currently awaiting governmental approval for human trials. The advent of a technique called mitochondrial replacement, which creates three-parent embryos, has brought survivors of Leigh syndrome several steps closer to having biological children without the mother’s diseased mitochondria.
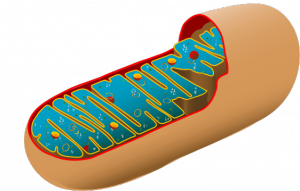
Mitochondria possess a small amount of DNA separate from the cell’s nuclear chromosomes, a remnant from when the organelles were free-living cells in the primordial world. Mutations in the mitochondrial DNA can result in severe, often fatal diseases – Leigh syndrome is just one manifestation of that. Without a functional way to produce cellular energy, entire organisms are at risk. In addition, because these mitochondrial diseases are passed from a mother to her offspring in the embryo, they are impossible to prevent and treat without altering the embryo.
Mitochondrial replacement combines two parents’ nuclear DNA, as in a normal embryo, and the mitochondria from a third, healthy donor egg. The embryo then grows into a child completely free of mitochondrial disease.
Of course, there are a multitude of safety and ethical issues preventing the immediate use of mitochondrial replacement. It is illegal in many countries to alter inheritable human DNA, though mitochondrial replacement would not result in any changes to nuclear DNA. Likewise, there are a variety of safety concerns for both mother and child, including catastrophic birth defects. Despite these hurdles, scientists are working to make the three-parent embryo a reality within the next two years.
The numerous functions of mitochondria make it apparent why this new therapy is an exciting advancement for the medical community. Mitochondria take in glucose and other nutrients and produce adenosine triphosphate (ATP), a molecule that retains and conveys chemical energy within cells. A constant supply of ATP is necessary for eukaryotic cells to survive, and thus for organisms, including humans, to function properly. Mitochondria can lose their ability to produce ATP via cellular respiration from changes in mitochondrial DNA.
However, mitochondria are not solely energy producers. The organelles play a major role in each cell’s metabolic pathways, and the 3,000 genes encoded in mitochondrial DNA regulate everything from detoxification to hormone synthesis. Mutations to mitochondrial DNA thus have far-reaching effects. It makes sense that Leigh syndrome and other mitochondrial diseases display such varied, serious symptoms; these conditions tend to negatively impact cells of the heart, brain, liver, kidneys, and skeletal muscles, resulting in severe symptoms ranging from developmental delay to cardiac disease.
Mitochondrial diseases are passed on from mother to child in the embryo. Though sperm do have mitochondria (they need energy to propel themselves to the egg), paternal mitochondria – along with all mitochondrial DNA – is usually lost immediately after fertilization. The mother’s egg, on the other hand, contains a multitude of mitochondria that become an intrinsic part of the embryo, and eventually a part of the mature organism. Mitochondria are matrilineal, meaning that they pass from mother to daughter completely unaltered for generations. This is unfortunate for women afflicted with Leigh syndrome, because they cannot naturally conceive healthy children. This is where mitochondrial replacement comes into play.
Scientists in the United States and abroad are testing several methods of mitochondrial replacement to create three-parent embryos and allow mothers with mitochondrial diseases to have their own biological children. One procedure, pronuclear transfer (PNT), completes in-vitro fertilization using the two primary parents’ gametes. At the same time, the father’s sperm is used to fertilize a donor egg with healthy mitochondria. Pronuclei, or the nuclei from the cells involved in fertilization, are removed from the primary parents and deposited into the second embryo. The embryo is eventually transferred to the mother with the hope of a successful birth of a disease-free baby.
Two additional methods of mitochondrial replacement include Maternal Spindle Transfer and Nuclear Genome Transfer, both of which utilize the mother’s nuclear DNA directly from the egg. The donor egg’s nuclear DNA is removed, and the mother’s nuclear DNA replaces the discarded donor DNA. The new egg with healthy mitochondria and the mother’s nuclear DNA is fertilized with sperm from the father in-vitro, and the egg is then transferred to the mother.
If mitochondrial replacement is legalized and is successful in clinical trials, one of these three methods — PNT, Maternal Spindle Transfer, or Nuclear Genome Transfer — will likely come to the surface as the safest and most effective way to prevent mitochondrial disease from passing through successive generations.
Of course there are a multitude of risks for women receiving in-vitro fertilization after mitochondrial replacement. First and foremost, pregnancy and delivery of a child is not guaranteed. The general in-vitro fertilization treatment is not entirely safe, and the addition of chemicals used in mitochondrial manipulation is speculated to increase the risk of harmful effects for the mother. Because mitochondrial replacement has yet to be tested in humans, scientists are unsure if the mother’s body will react negatively to the implanted three-parent embryo. Moreover, the three-parent child may not be perfectly healthy, and may instead be born with damaged physiology.
While it is true that three-parent children would avoid inheriting Leigh syndrome, they could potentially face other lethal complications. It is possible that birth defects and other negative reactions to the reagents used in the procedure will occur. In fact, mitochondrial replacement might even result in mitochondrial disease as a result of incomplete mitochondrial transfer or incompatibility.
A variety of diseases can also be caused by epigenetic changes that occur during mitochondrial replacement. These modifications do not affect DNA directly, but instead modify DNA expression through chemical reactions such as DNA methylation. Unfortunately, such detrimental epigenetic changes can be passed on to future generations.
Governments around the globe prohibit genetic modifications in humans for ethical reasons. Even the limited germ line modification entailed by mitochondrial replacement has caused disputes, both within the scientific community and amidst the public. On one side, opponents of the new therapy argue that if scientists can modify DNA in the embryo, there is nothing stopping them from creating “designer babies.” But in general, opposition to the three-parent embryo remains relatively quiet for now; proponents are looking ahead to the potential of mitochondrial replacement in helping patients of mitochondrial diseases live more fulfilling lives.
