In the sands of the Yukon Territory in Canada, a scientist found a beetle embedded with nano-scale diamonds. The insect was a small, brown member of the weevil family. It was plated with hollow scales, inside each of which expanses of nano-crystals had grown. Every diamond was placed exactly the same distance apart, yielding a formidably regular array that extended up and down and side to side, filling the insides of the beetle’s scales with a rigid, repeating matrix.
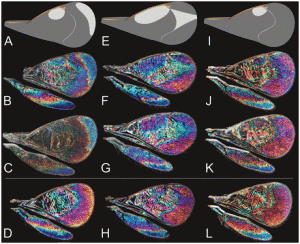
The insect unearthed in the Yukon sand had been preserved for over half a million years as a fossil. Yale researchers inspected the preserved material using high energy X-rays at Argonne National Laboratory in Chicago in order to study the configuration of crystals. Although the structures they discovered are especially beautiful and intriguing, diamond-filled scales are not unique: many creatures alive today grow identical or similar nano-size arrays. Animals grow these sorts of structures because they are optical powerhouses. By manipulating light, the crystals allow animals to produce brilliant colors that are otherwise unattainable.
Making Blue
Consider the color blue. There are no blue bears in the world. There are no blue crocodiles either. There are also no blue kangaroos, blue bumblebees, blue cats, or blue dogs.
There aren’t very many blue animals in the world, period, because blue is a difficult color to make. Most of the other colors of the rainbow arise straightforwardly in nature from chemicals, called pigments, that animals collect in their skin, feathers, and hair. These pigments include melanin and carotenoids, which reflect only certain colors of light. But as far as scientists can tell, there are no blue pigments in animals. They simply don’t exist.
But there are conspicuous examples of blue in nature, where animals have found a way around the challenge of creating the color. Creatures like blue jays and morpho butterflies have wings of such an intense, extravagant blue that every flap seems like a boast of success. These animals may deserve to brag: they have entirely circumvented the pigment problem by evolving a fundamentally new method of color production. Called structural color, the method uses extensive arrays of microscopically small structures, like crystals, to select and use only certain wavelengths of light.

Extracting a single wavelength from many wavelengths of light is no simple task. Just as picking up a single grain of rice can only be done with finely tapered chopsticks or especially small fingers, a wavelength can only be separated from other light waves by structures about the same size as the light itself. Since wavelengths of visible light are just a few hundred nanometers tall and wide, animals wishing to sort through them must acquire a mechanism that can operate on that scale.
That’s where the arrays of repeating structures come in handy. Each crystal block – be they diamonds or other shapes – are, not coincidentally, separated from one another by distances that are also a few hundred nanometers tall and wide. Sunlight hurtling from the sky towards the wings of a blue morpho butterfly arrives as a bundle of all the colors of the rainbow. Every wavelength, from red to violet, is present inside the white light. When all these rays strike the butterfly’s wings, the crystal arrays differentiate them by size. The wavelengths smaller than blue slip easily through the crystals. The wavelengths larger than blue bounce clumsily, too big to be reflected by the structures. The blue wavelengths, because of the precise configuration of the array, are reflected back out of the wings, and into my eyes and yours. Because we see only the blue wavelengths, the wings look blue to us.
In this way, a structure that naturally grows on the surfaces of several types of animals is able to dismantle one of the smallest, fastest-moving, most fundamental physical phenomena in the universe – light – into its component parts. This tactic of manipulating light is so sophisticated and so complex that humans have been unable to fully replicate butterfly wings’ optical properties. Part of what makes structural colors so intriguing to scientists is their ability to bend light in ways our technology cannot yet: animals that possess structural colors are masters of light.
How color grows
It seems inconceivable that a structure so precisely ordered and finely calibrated could be a biological creation. The configuration of diamonds seems more like the product of a machine than of a living thing. One would think that insects with structural colors must have elaborate instructions encoded in their genes detailing how to construct their edifices of crystal.
But here, as is often the case where biology is concerned, evolution has chosen the simplest solution. In fact, the animals that make structural colors do so with very little involvement on the part of their genes. That’s because evolution has prompted these creatures simply to coopt processes that already existed for other functions and convert them into mechanisms that make color.
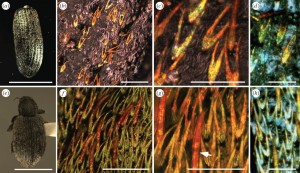
A modern weevil of the species Hypera diversipunctata, the species found in the Yukon. Image courtesy of Wikimedia.
Vinod Saranathan, GRD ’11 and now a research fellow at Nanyang Technological University in Singapore, was a lead researcher both in analyzing the fossil weevil and in determining how structural colors in butterflies grow. “There are two ancient features that make insects what they are,” he says, “that are being used to make color.”
The first is a strong, hard, translucent material called chitin. Almost all insects, whether or not they have structural colors, produce chitin. Because it is a resilient polymer, chitin does a good job of coating body surfaces and protecting soft tissue. Like melted cheese oozing over a pile of nachos, chitin adopts the shape of the surface underneath it. Eventually, it stops moving and hardens.
Insects have evolved a way to mold the chitin they already produce into optical nanostructures. To make the crystal-shaped molds for the chitin, they repurpose a second set of devices: membranes and proteins inside their cells.
Each cell on the top of a butterfly’s wing is ringed by a layer of flexible membrane. The membrane is thin, like a sheet of plastic wrap. Some of the proteins travel to the edges of the cell and stick to the membrane. Because they are relatively heavy, the membrane puckers slightly and sags inwards wherever a protein attaches. Because proteins clamp onto the membrane all over the cell, its surface becomes peppered with tiny hills and valleys.
Once chitin seeps over the wrinkled cell surface and fills in the folds, the chitin hardens and the cell dies. This leaves stacks of chitin structures molded into three-dimensional structures and separated by air, ready to receive light and make color.
Saranathan explains how this growth process can vary among animals, producing various crystal shapes and configurations. “Different families have slightly different proteins in their make-up,” Saranathan says, “so it’s only natural that different proteins would lead to different structures.”
Richard Prum, professor of Ecology and Evolutionary Biology at Yale and a world expert on structural color, notes that although they are built by animals, structural colors are not constructed according to the principles life usually follows.
“The cell does not specify the nanostructure in some kind of biological fashion,” says Prum. “It creates the physical conditions under which the nanostructure grows in predictable ways. So it uses physics in order to make what it needs to make the color.”
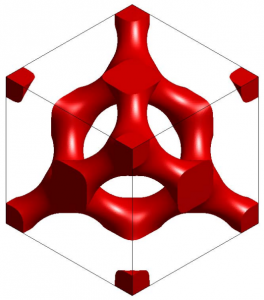
If the conditions are right, the chitin, commanded by nothing except the laws of physics, forms the structures on its own. Nothing is responsible for assembling the chitin into amazingly complex structures; it assembles itself. In this way, the construction of optical nanostructures is somewhat like a physical process elsewhere in nature, like the formation of clouds in the sky or ocean currents in the sea. The difference is that here, this purely physical process is attached to and dependent upon a living creature.
From Prum’s angle, making structural colors could be construed as a fairly “easy” process, evolutionarily speaking. That’s because evolution needs to modify just one process in order to send change cascading through the entire system. A slight difference in protein size affects the membrane’s curvature, which in turn drastically affects the chitin’s molded shape, which affects the color. In other words, a single small evolutionary modification to a set of proteins can lead to a large shift in the physical structures an animal ultimately produces.
“It’s like biology is rolling the ball up the hill,” Prum says, “and physics is the way it rolls down. You start it off, and then – BOOM – it just happens.”
What color can do
Although structural colors have been a subject of fascination for over a century at this point, most of the information we know about how they work and grow has been discovered in the last few decades. Apart from the obvious appeal sci-fi-esque nano-arrays combined with mesmerizing beauty, the phenomenon of structural color holds our attention because it still evades real understanding. Part of the ongoing mystique is the knowledge of how much more there is to learn.
For example, nothing we’ve learned so far tells us why these colors evolved in animals in the first place. To understand why they evolve, we must understand what they do. This is what Saranathan and Maria McNamara, a former Yale Research fellow who helped to analyze the weevil fossil, seek to do. In the case of bright blue birds and ostentatious butterflies, structural colors allow animals to make themselves attractive to mates or better at communicating from afar.
The crystals functioned somewhat differently for the fossilized weevil found in the Yukon. From afar, the weevil looked brown, but viewed up close, its colors were vibrant. The scientists hypothesize, therefore, that this weevil’s rather dull version of crystallized arrays functioned as a cryptic sign. To faraway predators, the beetle would have blended in with the brown sand in which it lived. But close up, to a potential mate, the beetle would have looked appealingly flashy.
Because of their usefulness and communication, structural colors have evolved many different times in many independent lineages. But exactly where and when they have arisen and disappeared in evolutionary history is still up for debate. To find out, McNamara, Saranathan, and others have turned to the fossil record. They hope to make use of the fact that even the most intricate structures can be preserved for hundreds of thousands of years in order to better understand which animals on the family tree evolved structural colors and which did not.
Besides their potential to inform us about past life history as well as future technological opportunity, structural colors have potential in the present as a bridge among the sciences. Understanding how they work requires physicists; understanding how they grow requires biologists; understanding how they are preserved over time requires chemists and geologists and paleontologists; understanding how they might be translated to human needs requires engineers. In an academic climate in which physicists are physicists and biologists are biologists and never the twain shall meet, the nano-scale arrays on the backs of butterflies are perhaps uniquely poised to stir scientific collaboration and incite interdisciplinary understanding.
Perhaps there may a secondary reason why everyone has a piece of the action where structural color research is concerned: not even the most jaded, wizened, hardened scientist can resist the allure of a morpho butterfly’s gorgeous blue.
Extra Reading:
- Geoffrey Edward Hill, Kevin J. McGraw, ed. (2006). “Anatomy, Physics, and Evolution of Structural Colors.” Bird Coloration: Mechanisms and measurements. Harvard University Press.
- Vinther, Jakob; Derek E.G. Briggs; Julia Clarke; Gerald Mayr; Richard O. Prum (2009). “Structural coloration in a fossil feather.” Biology Letters 6 (1): 128-31.
- Saranathan, Vinodkumar, et al. “Structure, function, and self-assembly of single network gyroid (I4132) photonic crystals in butterfly wing scales.” Proceedings of the National Academy of Sciences 107.26 (2010): 11676-11681.
About the Author: Julia Rothchild is a junior from Ann Arbor, Michigan majoring in Ecology and Evolutionary Biology. She is the magazine’s Articles Editor.
Acknowledgments: The author would like to thank Professor Richard Prum, Vinod Saranathan, Maria McNamara, and Emma Locatelli very much for their time and willingness to speak about their research.

