Throw a potpourri of transistors into the bathtub, add some soap, and out comes a fully-formed nanocomputer. Science fiction? Maybe not. Nanoscientists dream of coaxing electronic components to self-assemble into complex systems. Now, researchers at the University of Copenhagen have taken a major step towards making self-assembling electronics a reality.
In August, the researchers — many of whom were first year undergraduate students at the time of the work — reported that they had successfully induced randomly oriented molecular components to organize themselves into uniform sheets. At a time when electronic components are so small that it is a formidable challenge to position them accurately, self-assembly presents an elegant solution. Soap was the magical ingredient, forming thin films that sandwich the target molecules and precisely guide their orientation.
“Imagine you have a billion nanocomputers but they are all randomly oriented. You can’t harness the incredible computing power, nor can you ‘plug in’ the keyboard, the mouse, or the screen,” said Thomas Just Sørensen, lead investigator of the study and associate professor at the University of Copenhagen. “We need [the nanocomputers] to be orientated in the right way to each other, and that’s what our work seeks to accomplish.”
The promise of self-assembly
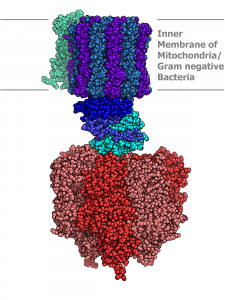
Nature provides inspiration for the flurry of work on self-assembly. From the aggregation of phospholipid molecules into cell membranes to the association of protein subunits into nanomachines that churn out energy when we metabolize sugars, nature creates elegant and intricate structures. These structures form spontaneously, without outside intervention — the tendency to self-assemble derives from the nature of the materials themselves.
Self-assembly holds great appeal at a time when electronic components have become incredibly small. Today, the transistors that make up computer chips are positioned and wired together on circuit boards using light, but this top-down approach is limited by the light’s wavelength. With bottom-up nanoscience, building blocks made of the right materials could do the hard work of assembly themselves.
Besides, self-assembling materials are more resilient than their traditional counterparts. If they can self-assemble once, it is generally safe to assume that they can self-assemble again upon suffering any damage. “If you break part of the material, there will be some kind of self-healing effect,” Sørensen said.
According to Sørensen, display technology is one field where self-assembling electronics promise a big splash. “All the technology in our smartphone is remarkably robust except for the screen,” he said. “There are no movable parts really. So you can hit it with a hammer, and if the screen doesn’t break, probably nothing else will.”
Soap: The magic ingredient
Sørensen and his team were after a substance that naturally organizes into well-defined layers. They wanted something that would not only sandwich thin films of electronic components, but would also align these components in the same direction. Here, they turned to soap.
This may seem like a surprising choice, since day-to-day experience suggests that mixing soap and circuitry is a bad idea. But the molecules that make up soap are excellent at forming layers (think: soap films). The water-loving ends of these molecules tend to stick together, as do their water-fearing tails. These films, the researchers hoped, would provide a regular template to guide the orientation of all molecular components added.
Not just any type of soap will work. As the team found, soap molecules found in common items such as shampoo and toothpaste lack the required rigidity to hold the molecular components tightly in place. Eventually, the group settled on a more grease-loving soap, benzalkonium chloride, which also happens to be an anti-fungal drug.
The team produced impeccably organized structures simply by mixing these soap particles with a range of dye molecules. The soap molecules quickly sought out other soap molecules and organized into thin films that effectively glued together layers of dye molecules. Even more impressive: the dye molecules oriented themselves in a common direction, lying flat on their sides just as a layer of bricks would pave a walkway.

Still, Sørensen estimates that self-assembling electronics are more than ten years away. In this proof-of-concept experiment, the researchers did not work with actual electronic components. Instead, they substituted similarly sized dye molecules. The nanomaterials they produced do provide insight into how soap organizes other molecular components. These materials may have interesting conducting properties in their own right — but they are not functioning electronic parts.
Even so, finding a material that can interact with other molecules to produce these elegant sheets is a leap forward, Sørensen said. Better yet, the scientists have already replicated their results. Working with a range of dyes with many different shapes, the team has observed self-assembly in 16 different nanomaterials. The Copenhagen scientists, among other researchers, are now chasing the next big break: translating these results to make functioning electronic parts.
A different philosophy to science education
The Copenhagen team’s work represents a breakthrough in science education as much as it does a breakthrough in science. This research grew out of coursework completed by first-year undergraduates as part of a laboratory class. Instead of conducting run-of-the-mill experiments, these freshmen enrolled in the university’s nanoscience program and had the opportunity to dedicate themselves to a modern engineering problem.
For Ida Boye, who took part in the fourth year of this research and who will be graduating this year, it was thrilling to realize that no one yet knew the answer to the questions that the team was tackling. “You have to think for yourself and try to come up with ideas, because there is no textbook telling you what is right and wrong,” Boye said.
Aske Gejl, one of the second batch of students now completing his master’s degree in nanoscience, credited this experience for his continued passion for research and inquiry. “The project still stands as one of the most important during my time at the university, as this was the first time I was trusted and enabled to aid in the progress of real scientific work. This only fueled my desire to strive for an academic career,” Gejl said.
Getting first-year students involved in research, pushing them to confront important questions, and having them see their work published in journals was a major achievement for the university staff, Sørensen said.
“The university is not just a teaching institute but also a research institute, and it’s important that [students] get to see this other side of the university,” he said.

Towards functional electronics
Sørensen is already looking ahead. As the classroom experiment moves into its sixth iteration, he hopes that the incoming batch of students will be able to build upon the existing work and produce functional self-assembling electronics.
Research teams elsewhere are hard at work trying to produce these layered devices by self-assembly. These groups typically work with larger compounds known as polymers instead of the smaller soap molecules that have worked so well for the Copenhagen teams. Sørensen explained that it might be easier to plug electrodes into materials made using long polymer strands, which take the form of boiled angel hair pasta: Each strand serves as a conducting wire, and it suffices to use an alligator clip that contacts the material at any two points. Soap films, on the other hand, require contacts small enough to pinch each individual film layer — just nanometers thick — and engineers have not yet developed electrodes that small.
Sørensen’s class, however, will continue to work with soap. He believes that there is value in pursuing this different path, especially for the first-year students who can afford to take bigger risks because they have less at stake.
One way or another, Sørensen said, self-assembly will deliver. “One day, we’ll be able to spread a thin layer of solar cells on the window and start generating solar power,” he said.
Self-assembling computers may still be a nanoscientist’s fantasy for now, Sørensen conceded. But as these remarkable films effortlessly organize tiny components with a dexterity that has eluded man’s best efforts, he is content to look on in wonder, marveling at how soap and self-assembly could shape the future of our most advanced electronics.
Cover Image: Art by Christina Zhang.