Cancer patients who have exhausted all traditional treatment options may still have hope for a new lease of life, thanks to their own immune systems.
Yale researchers have dramatically improved the conditions of certain patients with advanced incurable cancer, as reported in a Nature paper published this past November. They achieved this feat by blocking a molecular pathway that otherwise inactivated immune cells close to tumors. The team further identified biomarkers that could predict which patients were more likely to respond to the treatment.
“We have been trying immunotherapy for the last couple of decades. Until now, large trials have mostly been negative,” said Scott Gettinger, associate professor of medicine at the Yale Cancer Center, and one of the study’s authors.
Roy Herbst, professor of medicine at the Center and the study’s first author, explained that immunotherapy works because cancer cells have numerous mutations, making them different from normal body cells. Because cancer cells are technically foreign objects in the body, immune cells are able to attack them.

However, an immune response does not necessarily happen. One reason for this is that tumor cells make proteins that act like force fields, suppressing any nearby immune cells. Moreover, immune cells themselves make suppressive proteins when signals known as cytokines are present at high levels. This serves as the body’s safety mechanism to prevent an overactive immune response that could damage its own cells.
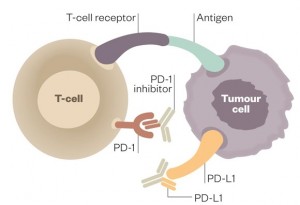
Programmed death-ligand 1 (PD-L1) is a notable example of this class of proteins that tones down the immune system. It binds to programmed cell death protein 1 (PD-1), a receptor found on the surface of T-cells, thereby preventing the activation of these immune cells.
Working with pharmaceutical company Genentech, Herbst and his team overcame this resistance mechanism by designing antibodies that bound to PD-L1, preventing it from interacting with PD-1. They injected these antibodies into patients every three weeks and observed a remarkable response. One in five patients improved thanks to the therapy, with the tumors of some patients shrinking within days. Importantly, the shrinkage was durable, unlike what often happens with chemotherapy where the tumor starts to grow once again.
What the Yale group has managed to achieve is “huge,” according to Kurt Schalper of the Yale School of Medicine, a clinician scientist in the field who was not involved in this work. Patients in this trial were patients who had already gone through all other available lines of treatment. “We are going from no treatment options to 20 percent response,” Schalper said.
Side effects are a common concern with immunotherapy. In reactivating the immune system, there is the possibility of generating an autoimmune response, when the body’s own cells are treated as foreign and are attacked. Fortunately, clinical trials show that adverse effects of the PD-L1 antibodies were generally mild, with few requiring medical treatment. Since the antibody is effective when administered alone, unlike other therapies which require a combination of several drugs, patients can expect to see less severe side effects, Schalper said.
Beyond developing this immunotherapy, researchers found predictive markers that can identify patients most likely to benefit from the treatment. Using these markers, doctors could administer the treatment in a selective manner such that the therapy would help up to half of patients given the drug, reducing the occurrence of unnecessary side effects.

Strikingly, some of these biomarkers are indicative of an activated immune response. In these patients, the immune system is primed to attack the tumor cells, but PD-L1 is holding it back. The antibody binds to PD-L1 and removes this barrier, allowing immune cells to perform their job.
Pharmaceutical companies are racing to develop drugs that will turn the body’s own defenses against aberrant cells. The PD-1/PD-L1 pathway is a popular target, and the Food and Drug Administration has already approved Merck’s anti-PD-1 drug for treatment of patients with advanced melanoma.
“This is a new paradigm in treating cancer,” Gettinger said. “In the last five years, the pessimism about immunotherapy has changed dramatically and now everyone is immensely interested. It used to be the last thing [doctors considered], but now it is the first.”
Gettinger spoke of one patient whose doctors had said he had three months left to live, but who participated in one of the first immunotherapy clinical trials five years ago. The patient did more than get better. His cancer seems to have disappeared completely.
“I’m fortunate enough to see these patients doing incredibly well five years later,” Gettinger said. “I wonder: Is it possible, is it actually possible, that we may never need to treat them again?”
